Abstract
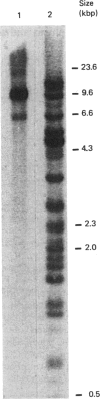
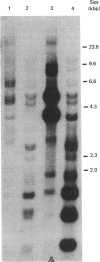
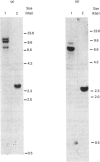
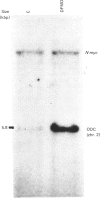
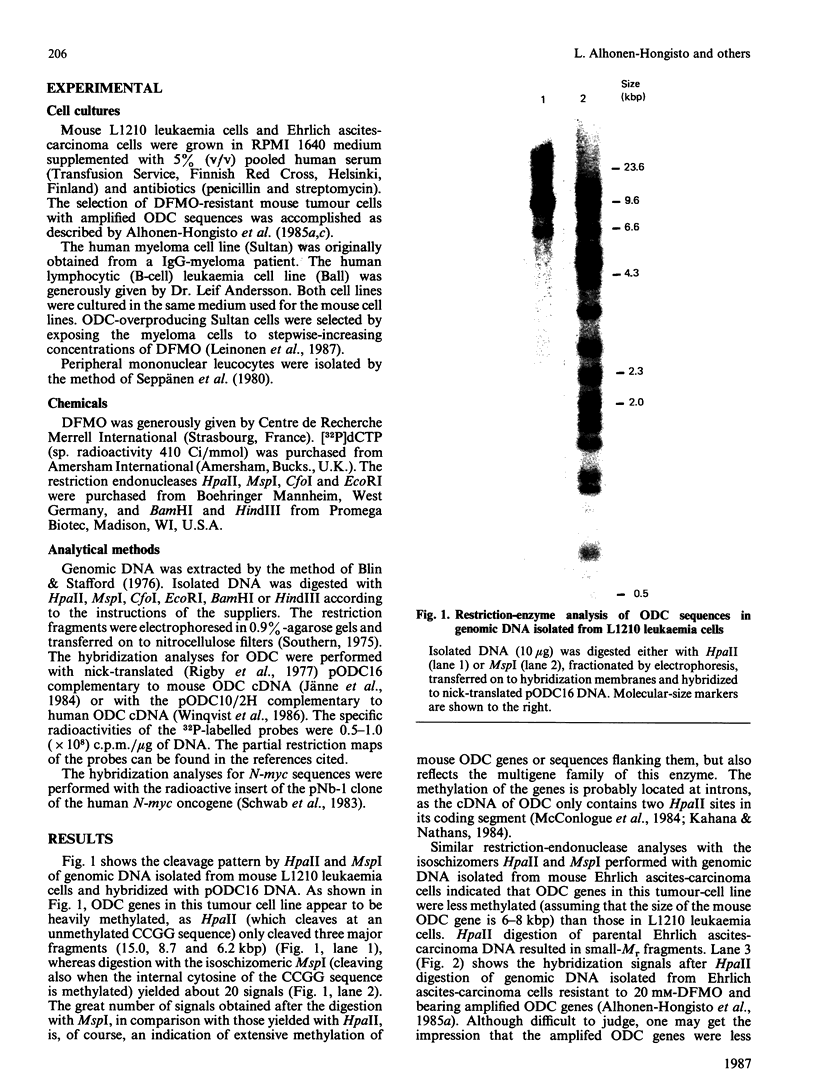
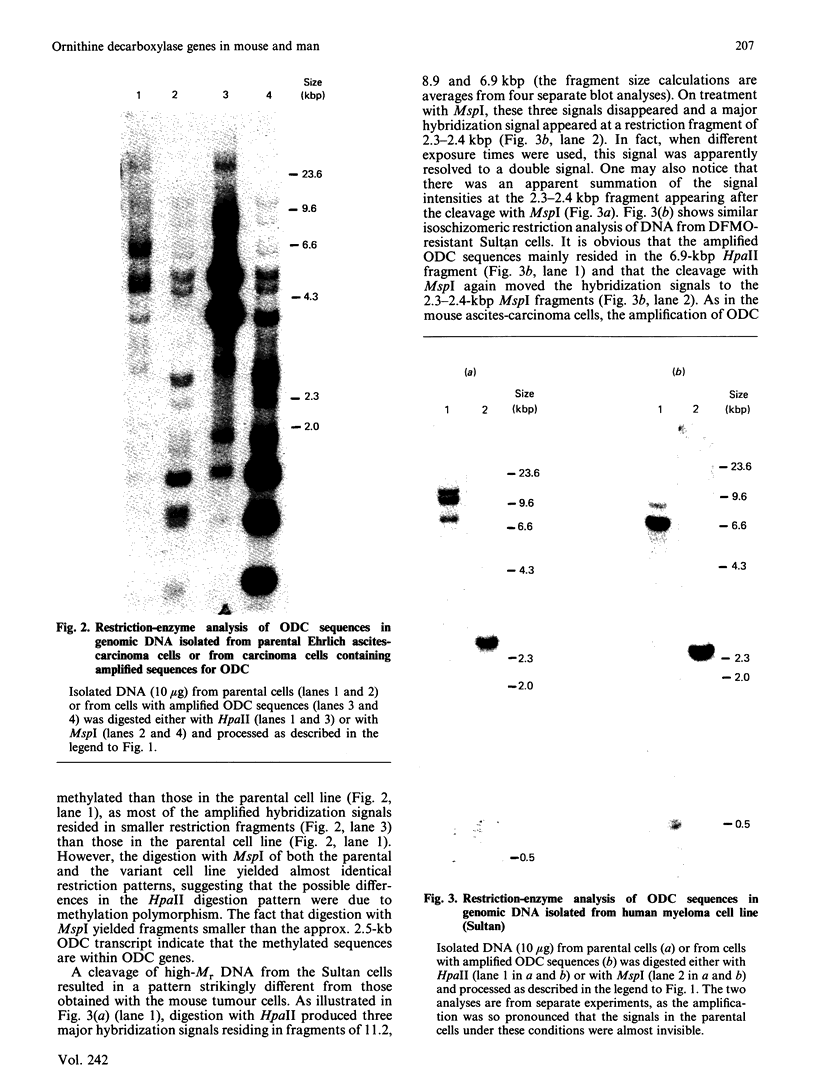
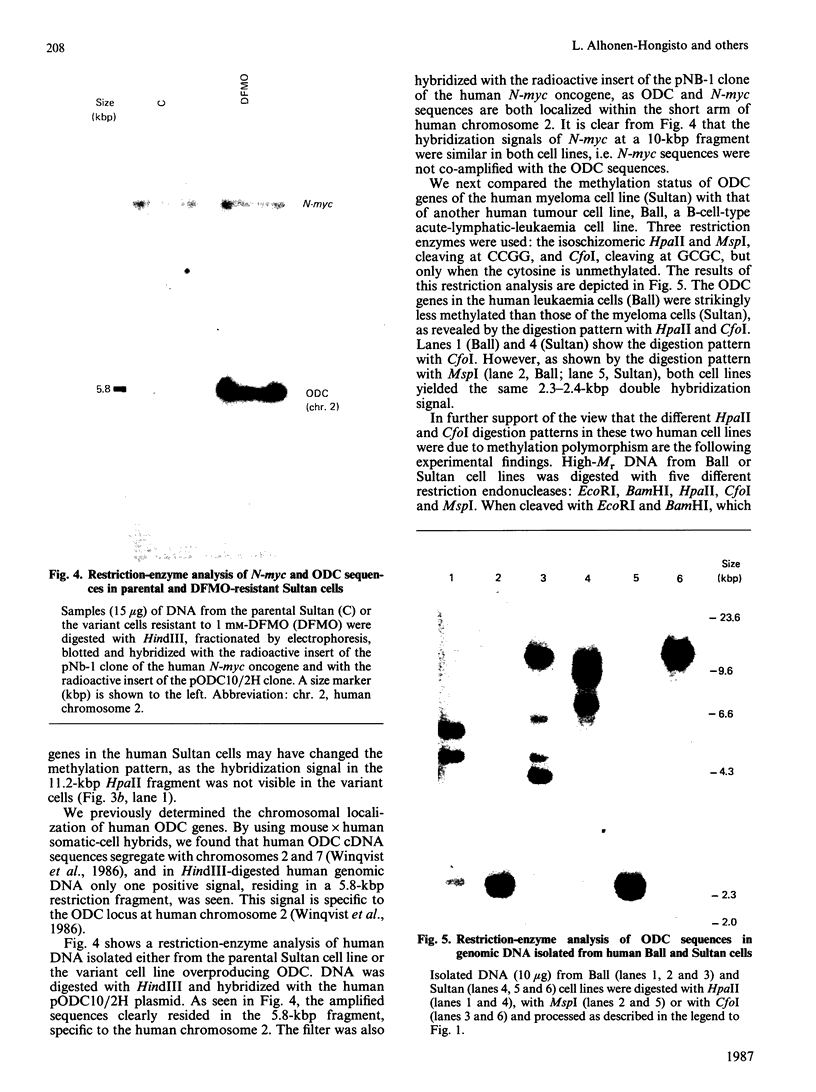
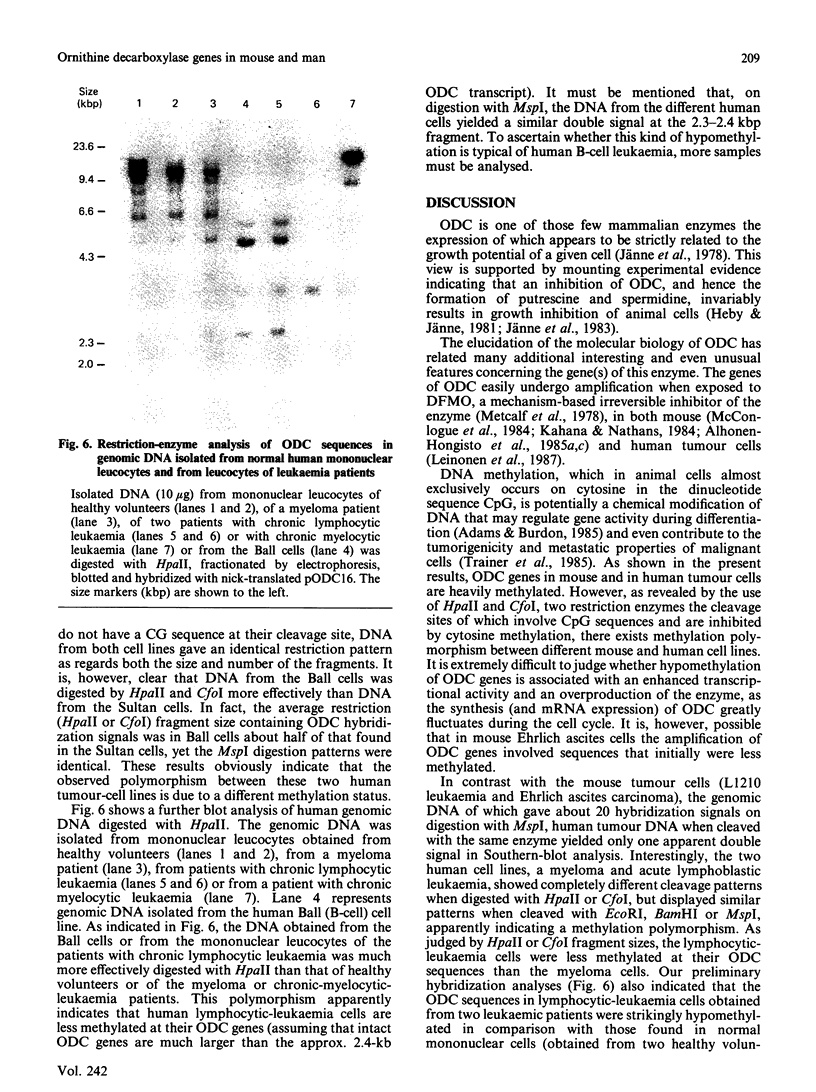
With the use of the isoschizomeric restriction endonucleases HpaII and MspI, we found that mouse tumour ornithine decarboxylase (ODC; EC 4.1.1.17) genes are extensively methylated. ODC genes in L1210 mouse leukaemia cells were apparently more methylated than in Ehrlich ascites carcinoma, as revealed by the use of HpaII endonuclease, yet the digestion of genomic DNA isolated from these two murine tumour cell lines with MspI, which cleaves at a CCGG sequence, also with internally methylated cytosine, resulted in an apparently identical restriction pattern. It is possible that the amplification of ODC genes in Ehrlich ascites-carcinoma cells in response to 2-difluoromethylornithine (DFMO) was associated with hypomethylation, or that less-methylated genes were amplified. A human myeloma (Sultan) cell line only revealed three separate hybridization signals when cleaved with HpaII. One of these signals was amplified under the pressure of DFMO. When cleaved with MspI, these three HpaII fragments disappeared and were replaced by a double signal of 2.3-2.4 kilobase-pairs (kbp) in size. The amplified ODC sequences in the Sultan myeloma cell line apparently originated from chromosome 2, as indicated by a unique hybridization signal in a 5.8 kbp HindIII fragment specific for the human ODC locus on chromosome 2. A comparison of different human cells, the Sultan myeloma, a lymphocytic B-cell leukaemia (Ball), normal mononuclear leucocytes and leucocytes obtained from leukaemia patients, revealed interesting differences in the methylation of ODC genes. The use of two restriction endonucleases (HpaII and CfoI), the cleavage site for both of which contains a CG sequence and which only cleave when cytosine is unmethylated, indicated that ODC genes in the lymphocytic leukaemia cells were much less methylated than those in the normal leucocytes or in the Sultan cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Kallio A., Sinervirta R., Jänne O. A., Gahmberg C. G., Jänne J. Tumourigenicity, cell-surface glycoprotein changes and ornithine decarboxylase gene pattern in Ehrlich ascites-carcinoma cells. Biochem J. 1985 Aug 1;229(3):711–715. doi: 10.1042/bj2290711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Kallio A., Sinervirta R., Seppänen P., Kontula K. K., Jänne O. A., Jänne J. Difluoromethylornithine-induced amplification of ornithine decarboxylase genes in Ehrlich ascites carcinoma cells. Biochem Biophys Res Commun. 1985 Jan 31;126(2):734–740. doi: 10.1016/0006-291x(85)90246-3. [DOI] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Sinervirta R., Jänne O. A., Jänne J. Gene expression of ornithine decarboxylase in L1210 leukaemia cells exposed to DL-2-difluoromethylornithine in the presence of cadaverine. Biochem J. 1985 Dec 1;232(2):605–607. doi: 10.1042/bj2320605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Hölttä E., Kallio A., Käpyaho K. Role of polyamines and their antimetabolites in clinical medicine. Spec Top Endocrinol Metab. 1983;5:227–293. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne O. A., Kontula K. K., Isomaa V. V., Bardin C. W. Ornithine decarboxylase mRNA in mouse kidney: a low abundancy gene product regulated by androgens with rapid kinetics. Ann N Y Acad Sci. 1984;438:72–84. doi: 10.1111/j.1749-6632.1984.tb38277.x. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen P., Alhonen-Hongisto L., Laine R., Jänne O. A., Jänne J. Human myeloma cells acquire resistance to difluoromethylornithine by amplification of ornithine decarboxylase gene. Biochem J. 1987 Feb 15;242(1):199–203. doi: 10.1042/bj2420199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Seppänen P., Alhonen-Hongisto L., Siimes M., Jänne J. Quantitation of methylglyoxal bis(guanylhydrazone) in blood plasma and leukemia cells of patients receiving the drug. Int J Cancer. 1980 Nov 15;26(5):571–576. doi: 10.1002/ijc.2910260508. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trainer D. L., Kline T., Mallon F., Greig R., Poste G. Effect of 5-azacytidine on DNA methylation and the malignant properties of B16 melanoma cells. Cancer Res. 1985 Dec;45(12 Pt 1):6124–6130. [PubMed] [Google Scholar]
- Winqvist R., Mäkelä T. P., Seppänen P., Jänne O. A., Alhonen-Hongisto L., Jänne J., Grzeschik K. H., Alitalo K. Human ornithine decarboxylase sequences map to chromosome regions 2pter----p23 and 7cen----qter but are not coamplified with the NMYC oncogene. Cytogenet Cell Genet. 1986;42(3):133–140. doi: 10.1159/000132266. [DOI] [PubMed] [Google Scholar]