Abstract
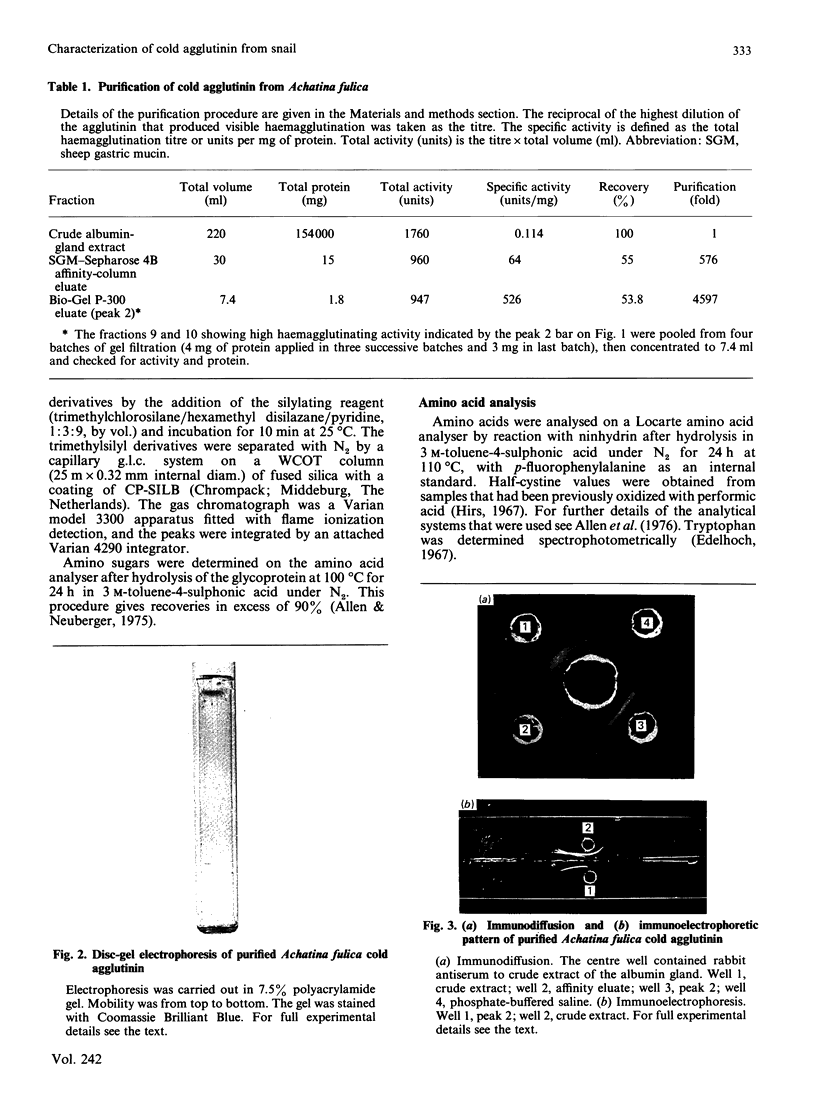
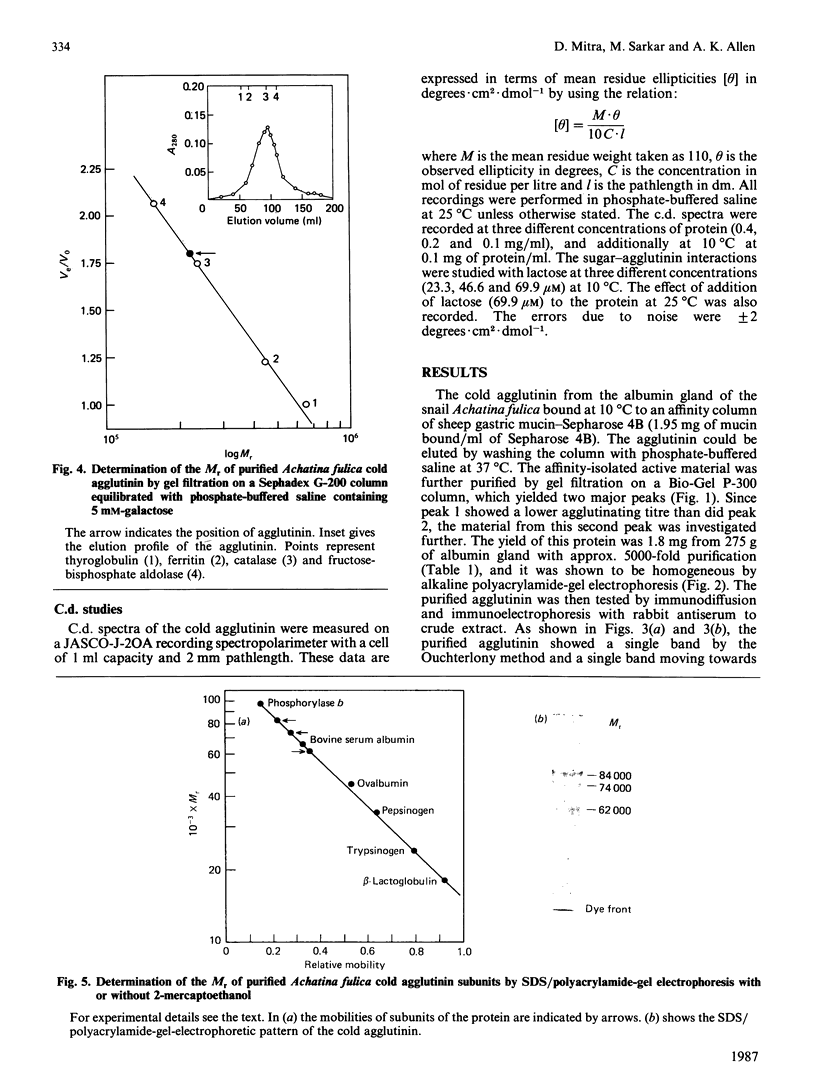
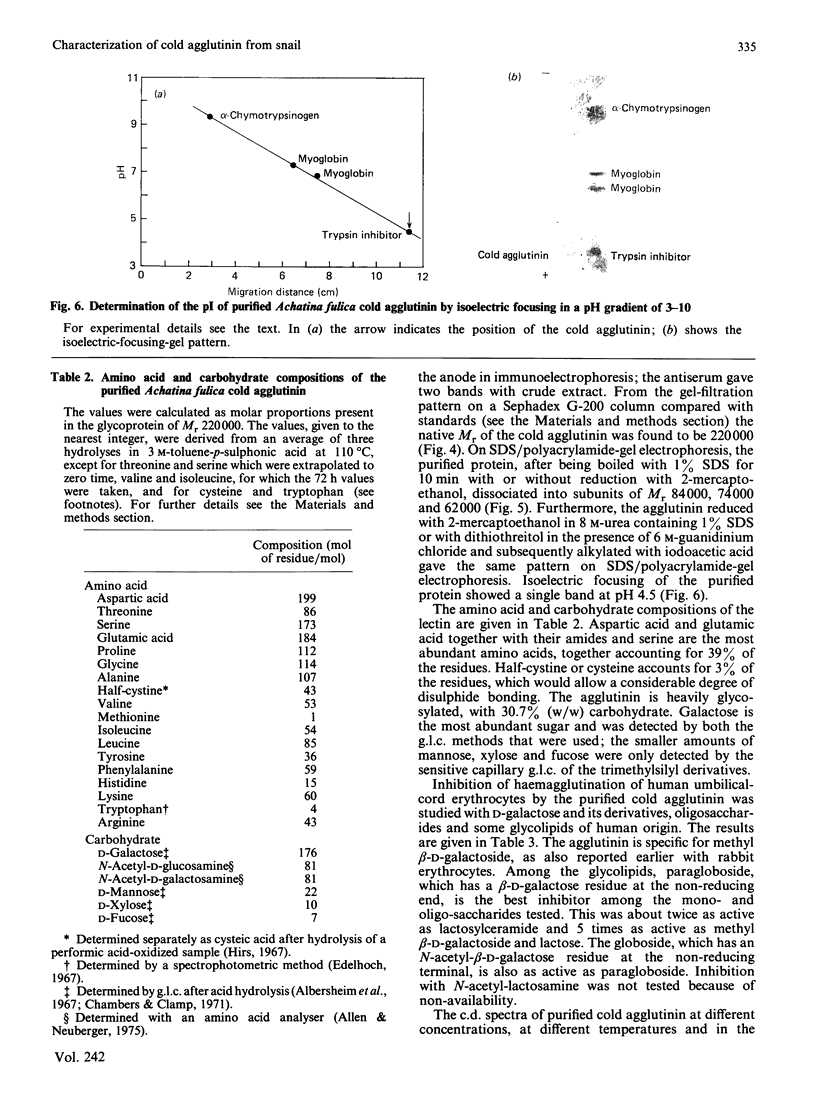
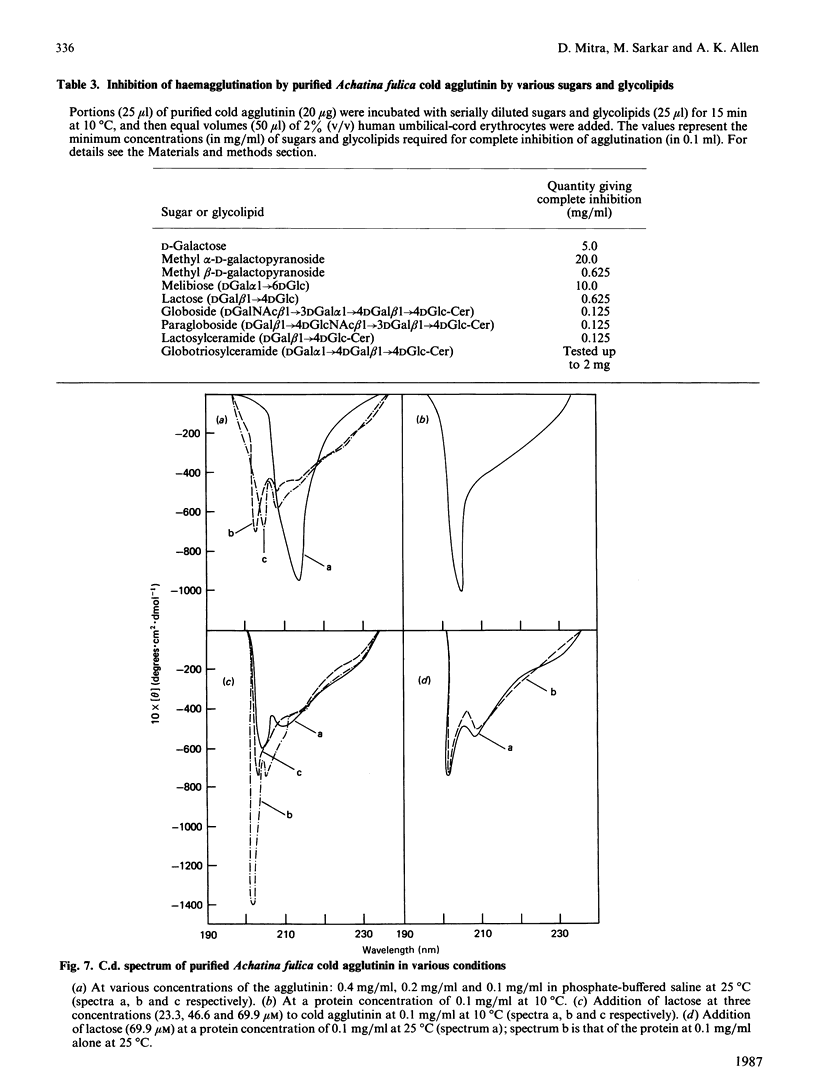
The cold agglutinin from the albumin gland of the snail Achatina fulica was purified to homogeneity by using sheep gastric mucin-Sepharose 4B as affinity column followed by gel filtration on Bio-Gel P-300. The homogeneity was checked by alkaline gel electrophoresis, immunodiffusion and immunoelectrophoresis. The purified cold agglutinin is a glycoprotein of native M2 220,000 consisting of three non-covalently bound subunits of Mr 84,000, 74,000 and 62,000 and having a pI value of 4.5. The predominant amino acids are aspartic acid and glutamic acid (or amides) and serine, which account for 39% of the residues. About 3% of the residues are half-cystine. The lectin is a glycoprotein with about 30.7% carbohydrate, the most abundant sugars being galactose, N-acetylgalactosamine and N-acetylglucosamine. Mannose, xylose and fucose are also present. The inhibition of agglutination of human umbilical-cord erythrocytes by the cold agglutinin is specific for methyl beta-D-galactoside and also for glycolipids present on cord erythrocytes. The c.d. data show only negative ellipticity values in the far-u.v. region for the protein at various concentrations and temperatures and also in the presence of the hapten lactose (at different concentrations), indicating the presence of a random-coil conformation in the agglutinin that varies according to temperature.
Full text
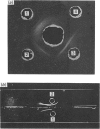
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A. Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem J. 1976 Apr 1;155(1):127–135. doi: 10.1042/bj1550127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The quantitation of glucosamine and galactosamine in glycoproteins after hydrolysis in p-toluenesulphonic acid. FEBS Lett. 1975 Dec 1;60(1):76–80. doi: 10.1016/0014-5793(75)80422-4. [DOI] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Feizi T. The blood group Ii system: a carbohydrate antigen system defined by naturally monoclonal or oligoclonal autoantibodies of man. Immunol Commun. 1981;10(2):127–156. doi: 10.3109/08820138109050693. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780(2):119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Kabat E. A. Purification and characterization of a blood-group A reactive hemagglutinin from the snail Helix pomatia and a study of its combining site. Biochemistry. 1969 Jul;8(7):2696–2705. doi: 10.1021/bi00835a002. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lönngren J., Goldstein I. F., Zand R. Circular dichroism studies on the alpha-D-galactopyranosyl binding lectin isolated from the seeds of Bandeiraea simplicifolia. Biochemistry. 1976 Jan 27;15(2):436–440. doi: 10.1021/bi00647a031. [DOI] [PubMed] [Google Scholar]
- MARSH W. L. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br J Haematol. 1961 Apr;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Pflumm M. N., Wang J. L., Edelman G. M. Conformational changes in concanavalin A. J Biol Chem. 1971 Jul 10;246(13):4369–4370. [PubMed] [Google Scholar]
- Père M., Bourrillon R., Jirgensons B. Circular dichroism and conformational transition of Dolichos biflorus and Robinia pseudoacacia lectins. Biochim Biophys Acta. 1975 May 30;393(1):31–36. doi: 10.1016/0005-2795(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Roelcke D. Cold agglutination. Antibodies and antigens. Clin Immunol Immunopathol. 1974 Jan;2(2):266–280. doi: 10.1016/0090-1229(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Rufo G. A., Jr, Singh J. P., Babcock D. F., Lardy H. A. Purification and characterization of a calcium transport inhibitor protein from bovine seminal plasma. J Biol Chem. 1982 Apr 25;257(8):4627–4632. [PubMed] [Google Scholar]
- Sarkar M., Bachhawat B. K., Mandal C. A new cold agglutinin from Achatina fulica snails. Arch Biochem Biophys. 1984 Aug 15;233(1):286–289. doi: 10.1016/0003-9861(84)90627-1. [DOI] [PubMed] [Google Scholar]
- Thomas M. W., Walborg E. F., Jr, Jirgensons B. Circular dichroism and saccharide-induced conformational transitions of wheat germ agglutinin. Arch Biochem Biophys. 1977 Jan 30;178(2):625–630. doi: 10.1016/0003-9861(77)90234-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wood E., Hounsell E. F., Langhorne J., Feizi T. Sheep gastric mucins as a source of blood-group-I and -i antigens. Biochem J. 1980 Jun 1;187(3):711–718. doi: 10.1042/bj1870711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaton R. W. Invertebrate lectins: I. Occurrence. Dev Comp Immunol. 1981 Summer;5(3):391–402. doi: 10.1016/s0145-305x(81)80052-3. [DOI] [PubMed] [Google Scholar]
- van Holst G. J., Martin S. R., Allen A. K., Ashford D., Desai N. N., Neuberger A. Protein conformation of potato (Solanum tuberosum) lectin determined by circular dichroism. Biochem J. 1986 Feb 1;233(3):731–736. doi: 10.1042/bj2330731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuik J. A., van Halbeek H., Kamerling J. P., Vliegenthart J. F. Primary structure of the low-molecular-weight carbohydrate chains of Helix pomatia alpha-hemocyanin. Xylose as a constituent of N-linked oligosaccharides in an animal glycoprotein. J Biol Chem. 1985 Nov 15;260(26):13984–13988. [PubMed] [Google Scholar]