Abstract
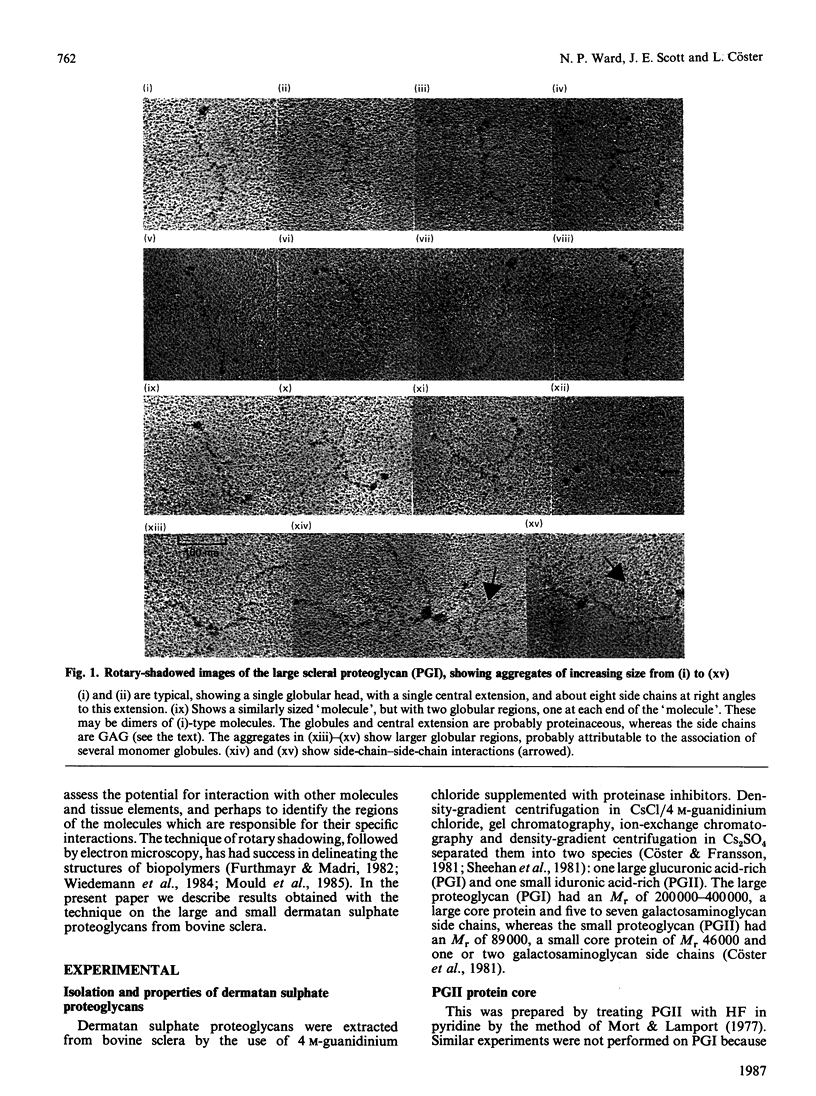
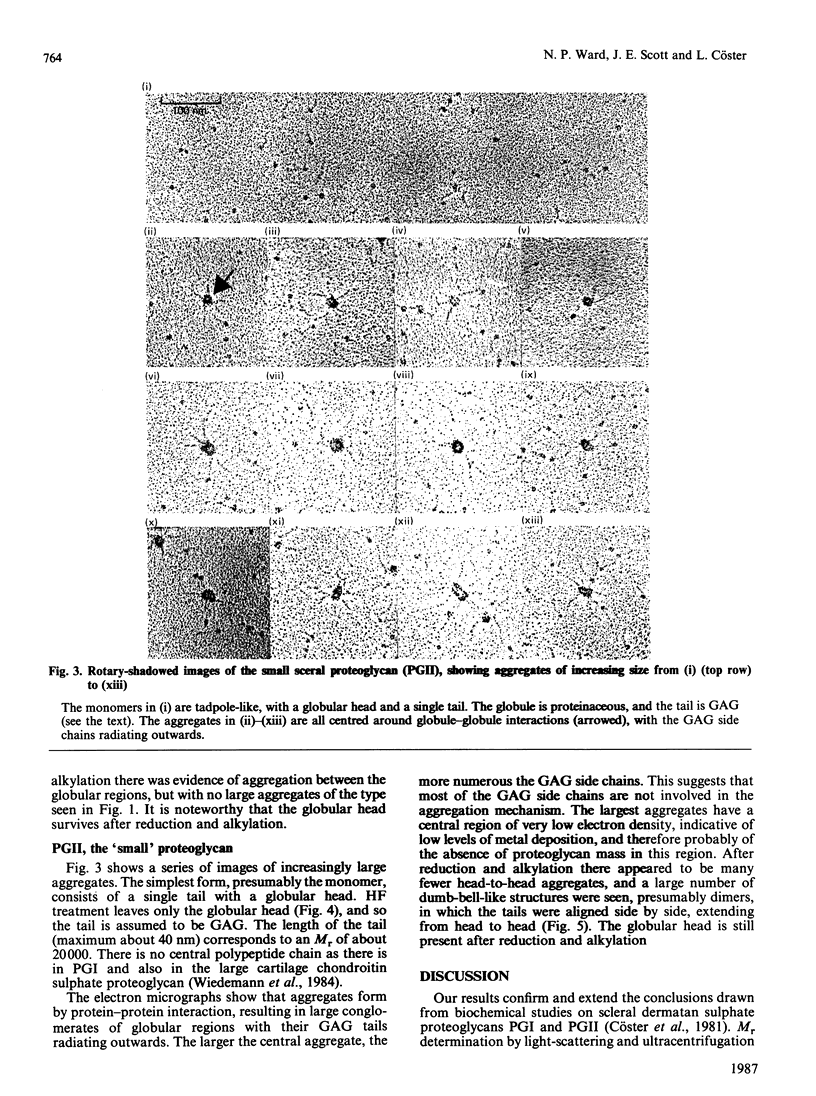
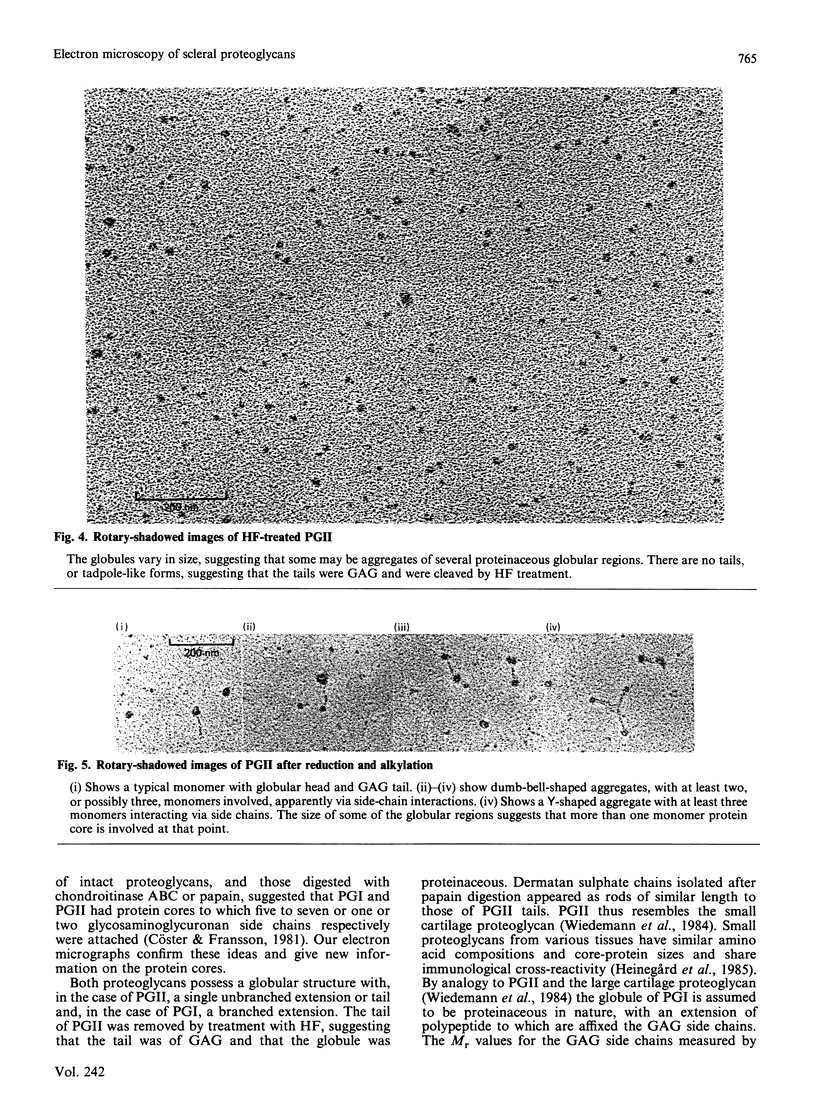
Two dermatan sulphate-containing proteoglycans from bovine sclera were examined by rotary shadowing and electron microscopy, and the results were compared with previous biochemical findings. Both the large iduronate-poor proteoglycan (PGI) and the small iduronate-rich proteoglycan (PGII) possessed a globular proteinaceous region. Whereas PGI had a branched extension from the globular region, with five to eight side chains attached to it, PGII had only a single tail, which was of glycosaminoglycuronan. PGII aggregated via globular-region interactions, which were much diminished by reduction and alkylation. PGI aggregated via side chains and globular-region interactions. Although a few PGI aggregates were large, and similar to the hyaluronan-cartilage proteoglycan aggregates [Weidemann, Paulsson, Timpl, Engel & Heinegård (1984) Biochem. J. 224, 331-333], hyaluronan did not cause enhanced aggregation. PGII is very similar in shape to the small cartilage chondroitin sulphate proteoglycan, whereas PGI somewhat resembles the large cartilage chondroitin sulphate proteoglycan, although with many fewer glycosaminoglycan side chains, and probably only one globular region as opposed to two in the cartilage proteoglycan.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cöster L., Carlstedt I., Malmström A. Isolation of 35S- and 3H-labelled proteoglycans from cultures of human embryonic skin fibroblasts. Biochem J. 1979 Dec 1;183(3):669–681. doi: 10.1042/bj1830669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A., Sheehan J., Nieduszynski I. A., Phelps C. F. Self-association of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Aug 1;197(2):483–490. doi: 10.1042/bj1970483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H., Madri J. A. Rotary shadowing of connective tissue macromolecules. Coll Relat Res. 1982 Jul;2(4):349–363. doi: 10.1016/s0174-173x(82)80025-3. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Holmes D. F., Kadler K. E., Chapman J. A. Mica sandwich technique for preparing macromolecules for rotary shadowing. J Ultrastruct Res. 1985 Apr;91(1):66–76. doi: 10.1016/0889-1605(85)90077-1. [DOI] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Hughes E. W. Proteoglycan-collagen relationships in developing chick and bovine tendons. Influence of the physiological environment. Connect Tissue Res. 1986;14(4):267–278. doi: 10.3109/03008208609017470. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-collagen interactions. Ciba Found Symp. 1986;124:104–124. doi: 10.1002/9780470513385.ch7. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I., Cöster L., Malmström A., Fransson L. A. Isopycnic-centrifugation studies in caesium chloride and in caesium sulphate on dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Dec 1;199(3):581–589. doi: 10.1042/bj1990581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N. P., Hulmes D. J., Chapman J. A. Collagen self-assembly in vitro: electron microscopy of initial aggregates formed during the lag phase. J Mol Biol. 1986 Jul 5;190(1):107–112. doi: 10.1016/0022-2836(86)90079-3. [DOI] [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]







