Abstract
Pancreatic cancer is an aggressive and metastatic tumor that lacks effective early detection and treatment methods. There is an urgent need to further understand its underlying molecular mechanisms and identify new biomarkers for early detection. Zinc, a critical trace element and catalytic cofactor, is tightly regulated within cells. ZIP4, a zinc transporter protein significantly overexpressed in human pancreatic cancer, appears to play a pivotal role in tumor development by modulating intracellular zinc concentration. This review highlights the role of ZIP4 in tumorigenesis, including its impact on pancreatic cancer growth, proliferation, migration, and drug resistance. ZIP4 exerts its effects by regulating zinc dependent transcriptional factors like CREB, STAT3, and ZEB1, resulting in upregulation of Cyclin D1, TP53INP1, ITGA3, CD44, ENT1 proteins, and miR-373. Moreover, ZIP4 mediates the miR373-PHLPP2-AKT signaling axis, which increases TGF-β expression. Coupled with CREB-activated macrophage catabolism-related genes SDC1 and DNM2, ZIP4 promotes cancer cachexia and supports amino acids to tumor cells under metabolic stress. Furthermore, ZIP4 facilitates bone resorption by osteoclasts via the RANKL-activated NF-κB pathway. A deeper understanding of these mechanisms may unveil potential targets for early diagnosis, prognosis assessment, and dietary recommendations for pancreatic cancer. These findings hold clinical significance not only for pancreatic cancer but also for other malignancies exhibiting heightened ZIP4 expression.
Keywords: Pancreatic cancer, ZIP4, Zinc, mechanisms, tumorigenesis
Introduction
Pancreatic cancer, notorious for its devastating impact, holds the highest mortality rate among cancers [1]. This grim prognosis is primarily due to its dense, proliferative stroma, a highly hypoxic and immunosuppressive tumor microenvironment, and the absence of effective screening tools and biomarkers. The maximum 5-year survival rate remains at a mere 10%, and by 2030, pancreatic cancer is projected to become the second leading cause of cancer-related death in the United States [2]. Zinc, an essential trace element, serves as an intracellular secondary messenger that regulates multiple signaling cascades, significantly influencing protein structure, enzyme activity, and gene regulation. Alterations in zinc concentrations in both the serum and malignant tissues of cancer patients have been consistently reported, underscoring its crucial role in cancer biology [3]. The intracellular free zinc concentration must be tightly regulated, typically maintained at pico-to-low nanomolar levels. Zinc transporters are pivotal in preserving intracellular zinc homeostasis by facilitating the uptake of extracellular zinc and its release into the cytoplasm [4]. Emerging evidence emphasizes the crucial role of zinc homeostasis and transport in cancer development, particularly in breast and pancreatic cancers [5,6]. Notably, ZIP4 stands out as the predominant upregulated zinc transporter protein and the most abundantly secreted exosomal protein in highly malignant pancreatic cancers [7,8]. This review focuses on the tumor-promoting effects of ZIP4 on pancreatic cancer proliferation, metastasis, drug resistance, and cachexia. By unraveling the underlying pathological mechanisms, we aim to identify novel therapeutic targets and biomarkers for early detection, treatment, tumor staging, and prognosis assessment in pancreatic cancer.
The pathophysiological role of zinc
Zinc, an essential trace element and catalytic cofactor, plays crucial physiological and pathological roles in maintaining normal cell growth and proliferation through its interaction with various transcription factors and metalloenzymes containing zinc finger motifs [9,10]. Zinc deficiency can induce apoptosis, hinder growth, delay bone development, lead to osteoporosis, impaired DNA synthesis, and immune system malfunction [11,12]. Dysregulated zinc transporter proteins often disrupt the function of zinc finger transcription factors, including cyclic adenosine monophosphate response element binding protein (CREB), CREB binding protein, and metal-responsive transcription factor-1, resulting in abnormal cellular activity, disrupting the balance of normal growth, and promoting uncontrolled transcription and cell proliferation. Additionally, zinc accelerates the malignant development of tumors as it is a component of vital enzymes, such as carbonic anhydrase and matrix metalloproteinases, both of which are involved in hypoxia, cell proliferation, angiogenesis, and metastasis [13,14]. However, excessive intracellular zinc is cytotoxic and can induce apoptosis, necessitating a homeostatic system to balance intracellular zinc uptake, storage, and efflux [15,16]. Two solute linkage carrier gene families, SLC30 and SLC39, encode zinc transporters Zinc Transporter (ZnT) and Zrt- and Irt-like Protein (ZIP), respectively, which play antagonistic roles in regulating zinc availability [17]. ZnTs reduce intracellular zinc by facilitating its efflux from cells or sequestering it into intracellular vesicles, while ZIPs increase intracellular zinc by promoting extracellular uptake and vesicular zinc release into the cytoplasm [4].
Altered zinc transporter expression has been associated with various cancers. Studies have reported reduced zinc concentrations in the serum and malignant tissues of patients with prostate cancer or hepatocellular carcinoma [18,19]. Breast cancer patients exhibit reduced serum zinc levels but increased intracellular zinc in malignant tissues due to low-level expression of ZnT1 [19-21]. Investigations also link breast cancer-associated proteins like ZIP6 to lymph node metastasis and estrogen-positive breast cancer [22], while ZIP10 has been implicated in breast cancer cell invasion [23]. Differences in serum and tumor tissue zinc concentrations across various cancers suggest tissue-specific zinc uptake mechanisms [4], collectively highlighting the crucial role of zinc transporter in cancer development. Among these transporters, ZIP4, encoded by the SLC39A4 gene, regulates intracellular zinc levels by absorbing dietary zinc and releasing it via vesicular compartments within intestinal epithelial cells [9]. The role of ZIP4 in tumors has gained attention (Table 1). Microarray analyses revealed overexpression of ZIP4 mRNA in human pancreatic cancer tissues. Li M et al. confirmed this through real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR) and immunohistochemical (IHC) staining, showing ZIP4 mRNA levels were approximately 5.5-fold higher in pancreatic cancer cell lines and tissues compared to human pancreatic ductal epithelial cells and surrounding normal tissues. Notably, ZIP4 expression was also elevated in pancreatic cancer tissues compared to chronic pancreatitis tissues [5]. Furthermore, ZIP4 emerged as the most abundantly secreted exosomal protein in highly malignant pancreatic cancers [8]. These findings suggest ZIP4 is a potential contributor to pancreatic cancer pathogenesis and may serve as a valuable diagnostic marker. Overexpressed ZIP4 supplies additional zinc to fuel tumor cell growth and plays a multifaceted role in pancreatic cancer, including proliferation, invasion, metastasis, tumor malignancy grade, cancer cachexia, bone loss, and drug resistance.
Table 1.
The role of ZIP4 in varies tumors
| Cancer | Expression of ZIP4 | Signal pathway | Outcome | Reference |
| Hepatocellular carcinoma | ↑ | ZIP4 regulates the expression of MMP2 and MMP9; down-regulates the expression of pro-apoptotic genes such as cystatinase-3, cystatinase-9, Bax; promotes the expression of Bcl-2. | ZIP4 promotes migration, invasion and inhibits apoptosis in hepatocellular carcinoma; and may promote re-entry of hepatocellular carcinoma cells into the cell cycle after release from G0/G1 blockade. | [76] |
| Ocarian cancer | ↑ | ZIP4 is involved in cellular activities related to HGSOC cancer stem cells through the ZIP4-HDAC4-VEGFA/ZIP4-NOTCH3-Jag-1 axis. | ZIP4 is functionally involved in CSC-associated cellular activities, including loss-of-nest resistance, colony formation, sphere formation, drug resistance, and LPA-induced EOC side populations in HGSOC cells. | [77,78] |
| Glioma | ↑ | ZIP4 levels are significantly associated with key genes for cell growth and angiogenesis in gliomas, such as VEGFA, MMP9, PDGFA, IGFBP2, IL-6 and IL-8. | High ZIP4 expression is significantly associated with higher glioma grade and shorter overall survival. | [79] |
| Gallbladder cancer | ↑ | ZIP4 up-regulate CDK4 and c-MET. | ZIP4 promotes Gallbladder cancer cell proliferation and migration and inhibits apoptosis. | [80] |
| Nasopharyngeal carcinma | ↑ | ZIP4 activates PI3K/Akt signaling pathway in Nasopharyngeal carcinma. | ZIP4 induces the EMT and promotes migration and invasion. | [81] |
| Non-small cell lung cancer | ↑ | ZIP4 activates the Snail-N-cadherin signaling pathway and promoting the EMT process. | ZIP4 promoted cell migration, invasion, and metastasis, and its expression level was negatively correlated with overall survival and progression-free survival in NSCLC patients. | [82] |
| Prostate cancer | ↓ | NA | ZIP4 has an inhibitory effect on the proliferation and invasion of prostate cancer. | [83] |
| Breast cancer | ↑ | NA | ZIP4 promotes tumorigenicity of breast cancer stem cells. | [84] |
MMP, matrix metalloproteinase; Bcl-2, B-cell lymphoma-2; HGSOC, High-Grade Serous Ovarian Cancer; HDAC4, Histone Deacetylase 4; VEGFA, Vascular Endothelial Growth Factor A; NOTCH3, Notch homolog 3; Jag-1, Jagged-1; CSC, Cancer Stem Cells; LPA, Lysophosphatidic Acid; EOC, Epithelial Ovarian Cancer; IGFBP2, Insulin-Like Growth Factor Binding Protein 2; IL-6, Interleukin-6; IL-8, Interleukin-8; CDK4, Cyclin-Dependent Kinase 4; c-MET, cellular-mesenchymal to epithelial transition factor; PI3K, Phosphatidylinositol 3-Kinase; EMT, Epithelial-Mesenchymal Transition; NSCLC, Non-Small Cell Lung Cancer; NA, Not Applicable.
ZIP4 promotes pancreatic cancer growth and proliferation and inhibits apoptosis
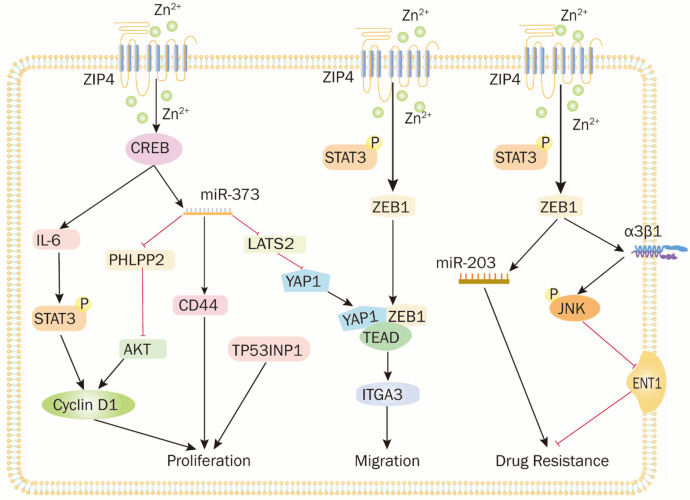
Zinc has been demonstrated to significantly impact cell proliferation by impacting DNA synthesis and cell cycle progression through deoxythymidine kinase and insulin-like growth factor-I [24]. In conditions of zinc deficiency, overexpressed ZIP4 exacerbates pancreatic cancer cell proliferation. Knockdown of ZIP4 significantly decreases tumor weight and volume in both subcutaneous and in situ models of the ASPC-1 pancreatic cancer cell line [25]. Zhang Y et al. using gene microarray, Reverse Transcription Quantitative Polymerase Chain Reaction, and western blot analysis, unraveled the signaling mechanism underlying ZIP4-mediated pancreatic cancer growth and proliferation [25]. They found that ZIP4 overexpression leads to elevated cyclin D1 levels, a critical regulator of the G1 to S-phase cell cycle transition, thereby promoting cell proliferation. ZIP4 overexpression also upregulates IL-6 expression via CREB activation, subsequently activating signal transducer and activator of transcription 3 (STAT3), leading to cyclin D1 elevating, and promoting pancreatic cancer cell proliferation and tumor progression. Additionally, ZIP4 overexpression significantly boosts microRNA-373 (miR-373) expression (by 6- to over 200-fold), which suppresses tumor-suppressor molecules like CD44, large tumor suppressor homologue 2 (LATS2), and TP53-inducible nuclear protein 1 (TP53INP1), further driving cancer cell proliferation [26]. Silencing CD44, LATS2, and TP53INP1 resulted in severe ascites, increased tumor weight and metastasis to the peritoneum, liver, spleen, and colon. Most tumors showed poor differentiation with tumor areas exceeding 80% compared to controls. PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2), a phosphatase involved in AKT signaling, is a target gene of miR-373 in pancreatic cancer. It inhibits CREB phosphorylation, establishing a CREB-miR-373-PHLPP2 feed-forward loop that activates the miR-373-PHLPP2-AKT-CyclinD1 signaling axis, driving pancreatic cancer growth [27]. Silencing miR-373 or blocking ZIP4 resulted in G0/G1 phase arrest, a decrease in the S-phase cell population, and significant reductions in tumor weight and size. Beyond promoting proliferation, ZIP4 also inhibits apoptosis. Under zinc-deficient conditions, ZIP4 knockdown increases susceptibility to apoptosis, as shown by flow cytometry analysis. ZIP4 overexpression prevents apoptosis by inhibiting the cleavage of caspase-7, caspase-9, and poly ADP-ribose polymerase, which are key components of apoptotic signaling. This resistance is partially mediated by the caspase-9 activator cytochrome C and the mitochondrial pathway pro-apoptotic protein Bax [28]. ZIP4 may also interfere with other apoptotic pathways, including the tumor necrosis factor (TNF) pathway via caspase-8 and the caspase-independent pathway managed by apoptosis-inducing factor. Impaired apoptosis contributes to both tumor progression and chemoresistance [29,30]. Defective apoptosis can lead to tumor progression and chemoresistance [31]. Given the reduced zinc levels in the serum and tumor tissues of pancreatic cancer patients, overexpressed ZIP4 enables pancreatic cancer cells to extract zinc from limited sources, supporting excessive proliferation and inhibiting apoptosis [32]. By balancing proliferation and apoptosis, ZIP4 plays a pivotal role in pancreatic cancer growth, highlighting the importance of zinc transport proteins in cancer cells (Figure 1).
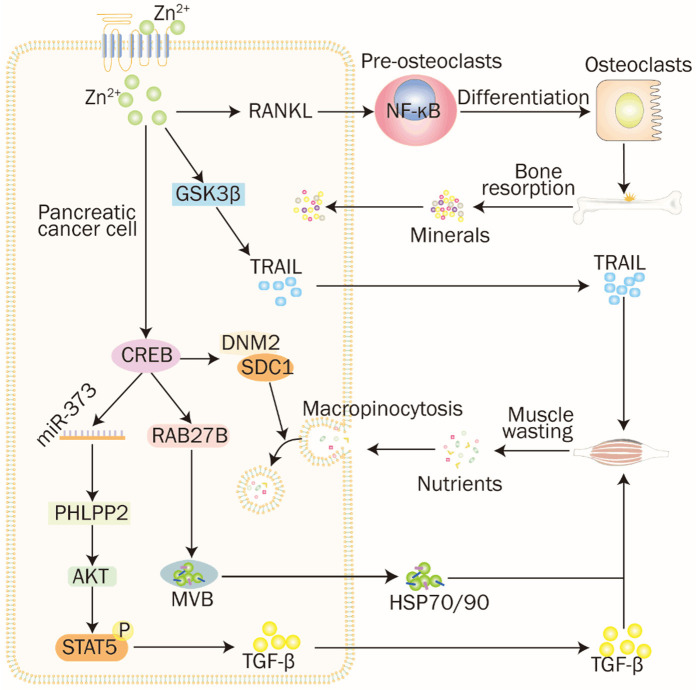
Figure 1.
The molecular signaling mechanisms and pathways of ZIP4 promoting pancreatic cancer cell proliferation, migration and drug resistance. (1) ZIP4 exerts a pro-muscle atrophic effect by phosphorylating STAT5 through the miR373-PHLPP2-AKT signaling axis to promote the expression of TGF-β. (2) ZIP4 increases the release of pancreatic cancer extracellular vesicles through the CREB-regulated RAB27B HSP70 and HSP90 stimulated the p38 MAPK signaling pathway and induced the expression of F-box protein and UBR2 in myotubes, leading to myofibrillar myosin heavy chain loss and myotube thinning. Meanwhile, ZIP4 activation of SDC1 and DNM2 via CREB and TRAIL secretion mediated through GSK3b could provide the required amino acid supply for tumor cell growth. (3) ZIP4 promotes the binding of RANKL to RANK on osteoclast membranes and facilitates bone resorption through activation of the NF-κB pathway, leading to decreased mineral density of bone tissue, increased bone crystallinity and bone strength loss.
ZIP4 promotes pancreatic cancer cell invasion and migration
Distant metastasis within 24 months post-surgery occurs in approximately 60% of pancreatic cancer patients [33,34], and is a major cause of death [35]. ZIP4 has been demonstrated as a crucial regulator of pancreatic cancer invasion and migration. Silencing ZIP4 in the ASPC-1 pancreatic cancer cell line notably reduced migration (~68%) and invasion (~81%), as confirmed by cell migration and invasion assays [25].
The degradation of the basement membrane is a key step in facilitating tumor cell migration and invasion, which begins with the breakdown of tight junctions between cells [36]. In normal epithelial cells, peripheral proteins like Zonula Occludens-1 (ZO-1) and transmembrane proteins like claudin-1 maintain cell-to-cell adhesion, preventing cancer cell migration [37]. Zinc has been shown to regulate the integrity of these tight junction proteins [38]. IHC staining analysis reveals that Zinc Finger E-Box Binding Homeobox 1 (ZEB1), a major transcription factor driving epithelial-mesenchymal transition (EMT), is overexpressed in pancreatic cancer tissues, while ZO-1 and claudin-1 levels are reduced compared to benign tissues [39]. Further studies indicate that overexpression of ZIP4 in pancreatic cancer cell lines upregulates the mesenchymal marker ZEB1 and reduces the epithelial markers ZO-1 and claudin-1. Through a ZEB1-dependent mechanism, ZIP4 amplifies downstream targets such as Focal Adhesion Kinase (an intracellular non-receptor tyrosine kinase associated with adhesion, migration and invasion) and Paxillin phosphorylate by downregulating claudin-1 and ZO-1, enhancing tumor invasion and metastasis independently of CREB [39].
EMT is a critical process for tumor metastasis, as it induces phenotypic changes that enable cancer cells to migrate, invade the basement membrane, and acquire stem cell-like characteristics [40,41]. ZEB1, apart from its role as a transcriptional repressor, can transition to a transcriptional activator by interacting with coactivators (Lef1, YAP1, P300, and Smad) regulating tumor progression via pathways like Hippo and Wnt [42-44]. The Hippo pathway plays an essential role in regulating cell shape, organ size, EMT, and tumorigenesis. YAP1, the major downstream effector of the Hippo pathway, has been shown to promote tumor growth in various cancers, including pancreatic, lung, and colon cancers. LATS2 can directly phosphorylate YAP1, inhibiting its activity through protein degradation. ZIP4 upregulates YAP1 expression by inhibiting LATS2 via miR-373, thereby activating downstream oncogenes [45-47]. YAP1 also acts as a co-activator by forming a complex with ZEB1, binding to the ITGA3 promoter region to enhance EMT plasticity, cellular adhesion, sphere formation, and tumor sphere formation [48]. ZEB1’s dual role - lower expression contributing to tumorigenesis and higher expression promoting metastasis - suggests its potential as a marker to distinguish between tumorigenesis and the EMT/metastasis stage [49]. Moreover, ZIP4 overexpression significantly elevates the Vascular Endothelial Growth Factor (VEGF), Neuropilin-1 (NRP-1, a receptor or co-receptor for specific isoforms of VEGF), matrix metalloproteinase (MMP)-9 and MMP-2 expression, which are critical molecules in angiogenesis, invasion, and metastasis within the ZIP4-initiated signaling cascade [50]. The specific regulatory mechanisms for NRP-1, VEGF, MMP-9, and MMP-2 in response to ZIP4 necessitate further investigation to comprehensively understand their role in ZIP4-mediated pancreatic cancer cell invasion and migration.
ZIP4 and drug resistance
Breakthroughs in pancreatic cancer treatment remain elusive, with gemcitabine-based monotherapy or combinations serving as the standard for advanced cases. Despite extensive exploration, no combination therapy has significantly outperformed gemcitabine alone [51]. Disease progression often leads to gemcitabine resistance, culminating in cancer recurrence [52]. Liu M et al. demonstrated that ZIP4 expression influences both the growth and chemoresistance of pancreatic cancer using a three-dimensional tumor culture model based on spheroids. When ZIP4 was overexpressed, MIA-PaCa-2 cell line enhanced resistance to cisplatin, 5-Fluorouracil (5-FU), and gemcitabine. suggesting ZIP4 as a potential predictor of chemoresistance in pancreatic cancer patients [51]. Their subsequent investigation delineated the regulatory cascade through which ZIP4 modulates drug resistance: ZIP4 phosphorylates STAT3, which subsequently upregulates ZEB1. ZEB1 increases the expression of integrin subunits ITGA1 and ITGB3, leading to the formation of integrin α3β1. This integrin complex downregulates equilibrium transporter protein 1 (ENT1) via c-Jun N-terminal Kinase mitogen-activated protein kinase (JNK MAPK) activation [51], reducing cellular uptake of gemcitabine and contributing to drug resistance [53]. Elevated ENT1 expression in pancreatic cancer typically indicates responsiveness to gemcitabine therapy, but ENT1 downregulation or deletion leads to gemcitabine resistance [54,55]. Additionally, ZEB1 inhibits miR-203, further contributing to drug resistance in pancreatic cancer [56].
Beyond integrin α3β1, integrin α2β1 enhances pancreatic cancer cell resistance to 5-FU through B-cell lymphoma 2 (Bcl-2) upregulation. ITGB1 promotes gemcitabine tolerance by activating Cdc42, while collagen/ITGA1 signaling plays a crucial role in gemcitabine tolerance [57,58] (Figure 2). These findings reveal unconventional pathways regulating metastasis and chemoresistance in pancreatic cancer and offer potential targets for future therapeutic strategies.
Figure 2.
ZIP4 accelerates the cancer cachexia of pancreatic cancer patients. ZIP4 activates downstream signaling pathways through zinc finger transcription factors CREB, STAT3, and ZEB1 leading to cyclinD1 up-regulate and through miR-373 negatively regulating tumor suppressor molecules TP53INP1, LATS2 and CD44 to promote pancreatic cancer proliferation and migration. And ZIP4 promotes the activation of MAP kinase JNK by integrin α3β1 through ZEB1 down-regulation of ENT1 leading to gemcitabine resistance.
ZIP4 and cancer cachexia
Cachexia, characterized by progressive muscle wasting, fat loss, reduced appetite, and weight loss [59-61], affects over 80% of pancreatic cancer patients and is resistant to refractory to conventional nutritional support [62,63]. This significant increase in muscle protein catabolism not only compromises patients’ quality of life but also impairs their tolerance and responsiveness to chemotherapy, thus contributing to tumor progression [64,65]. ZIP4, along with its upstream and downstream regulatory molecules, plays a crucial role in pancreatic cancer malignancy. Studies have shown that ZIP4 enhances muscle proteolysis, evidenced by significantly reduced tibialis anterior muscle mass and cross-sectional area in animal models with high ZIP4 expression compared to ZIP4 knockdown groups [27,66]. ZIP’s pro-muscle atrophy effect is mediated through the phosphorylation of STAT5 via the miR373-PHLPP2-AKT signaling axis, which promotes the expression of the soluble malignant plasma factor Transforming Growth Factor-β (TGF-β) [27]. CircANAPC7, a ZIP4-induced miR373 sponge, not only suppresses ZIP4-mediated cell proliferation through the miR373-PHLPP2-AKT-CyclinD1 signaling axis but also effectively ameliorates muscle atrophy in pancreatic cancer by inhibiting the miR373-PHLPP2-AKT-STAT5-TGF-β signaling axis. Mice with high circANAPC7 expression exhibited increased levels of myofibrillar protein and myosin heavy chain, alongside reduced expression of muscle atrophy markers such as Ubiquitin Protein Ligase E3 Component N-Recognin 2 (UBR2) protein, Muscle Atrophy F-box protein-1, and Muscle RING-finger protein-1. These mice also demonstrated improved grip strength, increased muscle weight, and enhanced cross-sectional area of muscle fibers [27]. This was supported by Kaplan-Meier survival curves, wherein circANAPC7 significantly increased median survival in pancreatic cancer xenograft mice compared to controls, underscoring the role of circANAPPC7 and ZIP4 in pancreatic cancer growth and malignancy.
Additionally, Yang J et al. demonstrated that ZIP4 enhances the release of heat shock proteins (HSP) HSP70 and HSP90 from pancreatic cancer extracellular vesicles (EVs) through CREB-regulated RAB27B, a GTPase necessary for controlling EV release [66]. These EV-associated proteins act as danger-associated molecular patterns, activating toll-like receptor 4 and stimulating the p38 MAPK pathway as well as the expression of F-box and UBR2 protein in myotubes, leading to thinning of myotubes and loss of myofibrillar myosin heavy chain [66]. Moreover, acetyl coenzyme A synthetase short-chain family member 2 (ACSS2), an enzyme involved in lipid synthesis, is overexpressed in pancreatic intraepithelial neoplasia lesions and pancreatic cancer tissues. ACSS2 promotes ZIP4 transcription by modifying the Ets Variant 4 promoter with H3K27ac histones. ZIP4, in turn, induces macropinocytosis through CREB-activated macropinocytosis catabolism-associated genes like Dynamin-2 (DNM2) and Syndecan-1 (SDC1), facilitating amino acids supply for tumor cell growth under metabolic stress states [67]. Notably, the downregulation of ZIP4 had a less pronounced effect on macropinocytosis compared to ACSS2 downregulation, suggesting that additional downstream signals of ACSS2, independent of ZIP4, may play a role in this process. ZIP4 also contributes to pancreatic cancer cachexia by secreting tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) 10 through the glycogen synthase kinase-b (GSK3b) pathway, leading to muscular atrophy [67].
In addition to its role in cachexia, ZIP4 has been implicated in bone loss associated with malignancy. ZIP4 promotes bone resorption by binding to Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL), a member of the TNF superfamily, which interacts with RANK on osteoclast membranes. This activates osteoclasts, and initiates the NF-κB pathway, resulting in reducing bone mineral density, increasing bone crystallinity, and compromising bone strength in situ xenograft mouse models [68].
Zinc and ZIP4 as therapeutic targets
Studies have shown that serum zinc levels are significantly reduced in pancreatic cancer patients, and zinc content in pancreatic tumor tissue is lower compared to adjacent normal tissue [32]. Furthermore, monitoring changes in trace element concentrations during pancreatic cancer treatment revealed that patients with decreasing zinc levels had higher mortality rates than those whose levels remained stable or increased [69]. Patients with larger reductions in zinc levels during treatment exhibited higher mortality rates compared to those with smaller reductions, and individuals whose zinc status shifted from normal to deficient had an increased risk of death [69]. The decline in zinc levels has been associated with cancer progression and poor outcomes, aligning with the overexpression of ZIP4 in human pancreatic cancer.
Numerous studies have highlighted the inhibitory impact of ZIP4 silencing on pancreatic cancer growth in subcutaneous and in situ xenograft models in nude mice [25,39,51]. ZIP4 silencing significantly reduced primary tumor weights, jaundice, peritoneal dissemination, hepatic and lung metastases, colon/intestinal obstruction, ascites, and weight loss, while also improving survival in in situ xenograft nude mice [25]. Histological analyses also indicated a reduction in tumor grade following ZIP4 silencing, potentially increasing tumor responsiveness to subsequent treatments. Consequently, combining ZIP4 short hairpin RNA with chemotherapy or radiotherapy may enhance the effectiveness of tailored therapies. Additionally, identifying ZIP4 expression profiles before and after pancreatic cancer treatment could guide the selection of initial and sequential ZIP4 therapies [25]. Tan X et al. were the first to demonstrat the utility of exosomal ZIP4 as a novel biomarker for pancreatic cancer diagnosis, leveraging clinical blood samples from healthy individuals and patients with malignant pancreatic cancer, benign pancreatic disease, or biliary tract disease. ZIP4 effectively distinguished malignant pancreatic cancer patients from both healthy controls (AUC=0.8931) and patients with benign pancreatic diseases (AUC=0.89) [8], highlighting ZIP4’s potential as a valuable diagnostic marker for pancreatic cancer.
Conclusion and perspectives
Pancreatic cancer is diagnosed late in over 80% of cases, hindering timely intervention due to the absence of distinct symptoms, rendering surgery unfeasible in most instances [70]. Approximately 15% of newly diagnosed cases present with non-metastatic and resectable tumors, with recurrence occurring within a year for most patients [71,72]. This highlights the urgent need for novel biomarkers that facilitate early diagnosis and treatment of pancreatic cancer. This review outlines various mechanisms wherein ZIP4 contributes to pancreatic cancer development and proposes avenues for further exploration. (1) We have summarized how ZIP4 promotes pancreatic cancer growth, proliferation, migration, chemoresistance, and cachexia. Some of its tumor-promoting effects are attributed to the disruption of tight junctions, promotion of EMT, and resistance to gemcitabine chemotherapy. However, studies have shown that ZIP4 also regulates the expression of MMP and increases resistance to other chemotherapeutic agents, such as 5-FU and cisplatin, although the exact mechanisms remain unclear. Therefore, further investigation is needed, such as how ZIP4 regulates MMP to promote tumor metastasis and the ZIP4’s synergistic or inhibitory effects in combination with gemcitabine-based therapies; (2) ZIP4’s biological effects are mediated through major transcriptional activators including CREB, STAT3, and ZEB1, making them potential therapeutic targets. However, effective methods for precisely targeting ZIP4 or its downstream transcriptional activators and delivering such therapies need further development; (3) Given that zinc levels in ZIP4-overexpressing tumor cells remain lower than those in surrounding normal pancreatic tissue, we hypothesize that this may be due to excessive zinc consumption required for tumor survival and growth. However, the exact reasons for this phenomenon remain to be explored. It’s generally believed that zinc can exert anti-tumor activity by improving immune function, reducing oxidative stress, and promoting DNA damage repair [73,74]. Thus, exploring strategies to harness high levels of zinc in pancreatic cancer to induce anti-tumor activity might be more beneficial than directly silencing ZIP4; (4) Given ZIP4’s role in zinc uptake, heavy meat diets, particularly red and double-cooked meat, cause excessive zinc absorption in humans, which a recognized risk factor for pancreatic cancer development [75]. This suggests that dietary recommendations could be valuable for pancreatic cancer patients. Carefully designed, large-scale cohort studies are necessary to evaluate zinc status and its implications for early diagnosis and prognosis in patients with pancreatic cancer. In conclusion, Zinc and ZIP4 may serve as promising diagnostic, therapeutic, and prognostic biomarkers for pancreatic cancer. Targeting ZIP4 with innovative treatments may offer promising treatment strategies for not only pancreatic cancer but also other malignancies displaying elevated ZIP4 expression.
Acknowledgements
We gratefully acknowledged the National Natural Science Foundation of China for supporting our research (Grant No. 81871324).
Disclosure of conflict of interest
None.
References
- 1.Yang J, Ren B, Yang G, Wang H, Chen G, You L, Zhang T, Zhao Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–321. doi: 10.1007/s00018-019-03278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med. 2020;17:612–625. doi: 10.20892/j.issn.2095-3941.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 7.Yang J, Zhang Y, Cui X, Yao W, Yu X, Cen P, Hodges SE, Fisher WE, Brunicardi FC, Chen C, Yao Q, Li M. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr Mol Med. 2013;13:401–409. [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H, Liu P, Wu Y, Meng X, Wu M, Han J, Tan X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109:2946–2956. doi: 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130(Suppl):1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A. Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone. 2006;38:181–198. doi: 10.1016/j.bone.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Juhász M, Chen J, Lendeckel U, Kellner U, Kasper HU, Tulassay Z, Pastorekova S, Malfertheiner P, Ebert MP. Expression of carbonic anhydrase IX in human pancreatic cancer. Aliment Pharmacol Ther. 2003;18:837–846. doi: 10.1046/j.1365-2036.2003.01738.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Hypoxia and angiogenesis in pancreatic cancer. ANZ J Surg. 2006;76:830–842. doi: 10.1111/j.1445-2197.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 16.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 17.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 18.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarty PK, Ghosh A, Chowdhury JR. Zinc in human malignancies. Neoplasma. 1986;33:85–90. [PubMed] [Google Scholar]
- 20.Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Mulay IL, Roy R, Knox BE, Suhr NH, Delaney WE. Trace-metal analysis of cancerous and noncancerous human tissues. J Natl Cancer Inst. 1971;47:1–13. [PubMed] [Google Scholar]
- 22.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–59. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee R, Woo W, Wu B, Kummer A, Duminy H, Xu Z. Zinc accumulation in N-methyl-N-nitrosourea-induced rat mammary tumors is accompanied by an altered expression of ZnT-1 and metallothionein. Exp Biol Med (Maywood) 2003;228:689–696. [PubMed] [Google Scholar]
- 25.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Yang J, Cui X, Chen Y, Zhu VF, Hagan JP, Wang H, Yu X, Hodges SE, Fang J, Chiao PJ, Logsdon CD, Fisher WE, Brunicardi FC, Chen C, Yao Q, Fernandez-Zapico ME, Li M. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol Med. 2013;5:1322–1334. doi: 10.1002/emmm.201302507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X, Yang J, Liu M, Zhang Y, Zhou Z, Luo W, Fung KM, Xu C, Bronze MS, Houchen CW, Li M. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2-AKT-TGF-β signaling axis in pancreatic cancer. Gastroenterology. 2022;162:2004–2017. e2002. doi: 10.1053/j.gastro.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Zhang Y, Yang J, Sun X, Hagan JP, Guha S, Li M. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle. 2014;13:1180–1186. doi: 10.4161/cc.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 30.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 31.Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byeon S, du Toit-Thompson T, Hipperson L, Maloney S, Wenzel R, Gill AJ, Samra JS, Mittal A, Sahni S. Serum and tissue metallome of pancreatic ductal adenocarcinoma. Cancer Sci. 2024;115:1446–1458. doi: 10.1111/cas.16124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Metastasis-suppressor genes: a review and perspective on an emerging field. J Natl Cancer Inst. 2000;92:1717–1730. doi: 10.1093/jnci/92.21.1717. [DOI] [PubMed] [Google Scholar]
- 36.Martin TA. The role of tight junctions in cancer metastasis. Semin Cell Dev Biol. 2014;36:224–231. doi: 10.1016/j.semcdb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci. 2013;14:18148–18180. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyoshi Y, Tanabe S, Suzuki T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am J Physiol Gastrointest Liver Physiol. 2016;311:G105–116. doi: 10.1152/ajpgi.00405.2015. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, Yang J, Zhang Y, Zhou Z, Cui X, Zhang L, Fung KM, Zheng W, Allard FD, Yee EU, Ding K, Wu H, Liang Z, Zheng L, Fernandez-Zapico ME, Li YP, Bronze MS, Morris KT, Postier RG, Houchen CW, Yang J, Li M. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and Claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin Cancer Res. 2018;24:3186–3196. doi: 10.1158/1078-0432.CCR-18-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G EMT International Association (TEMTIA) Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tièche CC, Peng RW, Dorn P, Froment L, Schmid RA, Marti TM. Prolonged pemetrexed pretreatment augments persistence of cisplatin-induced DNA damage and eliminates resistant lung cancer stem-like cells associated with EMT. BMC Cancer. 2016;16:125. doi: 10.1186/s12885-016-2117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498. doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosmaninho P, Mükusch S, Piscopo V, Teixeira V, Raposo AA, Warta R, Bennewitz R, Tang Y, Herold-Mende C, Stifani S, Momma S, Castro DS. Zeb1 potentiates genome-wide gene transcription with Lef1 to promote glioblastoma cell invasion. EMBO J. 2018;37:e97115. doi: 10.15252/embj.201797115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Cho EJ, Kim ST, Youn HD. CtBP represses p300-mediated transcriptional activation by direct association with its bromodomain. Nat Struct Mol Biol. 2005;12:423–428. doi: 10.1038/nsmb924. [DOI] [PubMed] [Google Scholar]
- 45.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Kim DH, Shah SR, Kim HN, Kshitiz, Kim P, Quiñones-Hinojosa A, Levchenko A. Switch-like enhancement of epithelial-mesenchymal transition by YAP through feedback regulation of WT1 and Rho-family GTPases. Nat Commun. 2019;10:2797. doi: 10.1038/s41467-019-10729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P, Pešl M, Caluori G, Pagliari S, Martino F, Maceckova Z, Hajduch M, Sanz-Garcia A, Pugno NM, Stokin GB, Forte G. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Zhang Y, Yang J, Zhan H, Zhou Z, Jiang Y, Shi X, Fan X, Zhang J, Luo W, Fung KA, Xu C, Bronze MS, Houchen CW, Li M. Zinc-dependent regulation of ZEB1 and YAP1 coactivation promotes epithelial-mesenchymal transition plasticity and metastasis in pancreatic cancer. Gastroenterology. 2021;160:1771–1783. e1771. doi: 10.1053/j.gastro.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Lu X, Huang L, Wang W, Jiang G, Dean KC, Clem B, Telang S, Jenson AB, Cuatrecasas M, Chesney J, Darling DS, Postigo A, Dean DC. Different thresholds of ZEB1 are required for Ras-mediated tumour initiation and metastasis. Nat Commun. 2014;5:5660. doi: 10.1038/ncomms6660. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Chen C, Yao Q, Li M. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther. 2010;9:236–242. doi: 10.4161/cbt.9.3.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H, Ding K, Tian X, Yang Z, Fung KA, Edil BH, Postier RG, Bronze MS, Fernandez-Zapico ME, Stemmler MP, Brabletz T, Li YP, Houchen CW, Li M. ZIP4 increases expression of transcription factor ZEB1 to promote integrin α3β1 signaling and inhibit expression of the gemcitabine transporter ENT1 in pancreatic cancer cells. Gastroenterology. 2020;158:679–692. e671. doi: 10.1053/j.gastro.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bapiro TE, Richards FM, Goldgraben MA, Olive KP, Madhu B, Frese KK, Cook N, Jacobetz MA, Smith DM, Tuveson DA, Griffiths JR, Jodrell DI. A novel method for quantification of gemcitabine and its metabolites 2’,2’-difluorodeoxyuridine and gemcitabine triphosphate in tumour tissue by LC-MS/MS: comparison with (19)F NMR spectroscopy. Cancer Chemother Pharmacol. 2011;68:1243–1253. doi: 10.1007/s00280-011-1613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26:85–110. doi: 10.1007/s10555-007-9044-4. [DOI] [PubMed] [Google Scholar]
- 54.Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740–747. doi: 10.1593/neo.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, Dicker AP, Mackey JR. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 56.Meidhof S, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M, Schüler J, Berthold M, Weber A, Burk U, Lübbert M, Puhr M, Culig Z, Wellner U, Keck T, Bronsert P, Küsters S, Hopt UT, Stemmler MP, Brabletz T. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med. 2015;7:831–847. doi: 10.15252/emmm.201404396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang D, Tang Y, Fu H, Xu J, Hu Z, Zhang Y, Cai Q. Integrin β1 promotes gemcitabine resistance in pancreatic cancer through Cdc42 activation of PI3K p110β signaling. Biochem Biophys Res Commun. 2018;505:215–221. doi: 10.1016/j.bbrc.2018.09.061. [DOI] [PubMed] [Google Scholar]
- 58.Gharibi A, La Kim S, Molnar J, Brambilla D, Adamian Y, Hoover M, Hong J, Lin J, Wolfenden L, Kelber JA. ITGA1 is a pre-malignant biomarker that promotes therapy resistance and metastatic potential in pancreatic cancer. Sci Rep. 2017;7:10060. doi: 10.1038/s41598-017-09946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 60.Martignoni ME, Kunze P, Friess H. Cancer cachexia. Mol Cancer. 2003;2:36. doi: 10.1186/1476-4598-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care. 2016;19:188–198. doi: 10.1097/MCO.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 62.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Ronga I, Gallucci F, Riccardi F, Uomo G. Anorexia-cachexia syndrome in pancreatic cancer: recent advances and new pharmacological approach. Adv Med Sci. 2014;59:1–6. doi: 10.1016/j.advms.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 65.Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30:489–501. doi: 10.1101/gad.276733.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Zhang Z, Zhang Y, Ni X, Zhang G, Cui X, Liu M, Xu C, Zhang Q, Zhu H, Yan J, Zhu VF, Luo Y, Hagan JP, Li Z, Fang J, Jatoi A, Fernandez-Zapico ME, Zheng L, Edil BH, Bronze MS, Houchen CW, Li YP, Li M. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology. 2019;156:722–734. e726. doi: 10.1053/j.gastro.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z, Ren Y, Yang J, Liu M, Shi X, Luo W, Fung KM, Xu C, Bronze MS, Zhang Y, Houchen CW, Li M. Acetyl-Coenzyme A synthetase 2 potentiates macropinocytosis and muscle wasting through metabolic reprogramming in pancreatic cancer. Gastroenterology. 2022;163:1281–1293. e1281. doi: 10.1053/j.gastro.2022.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Q, Sun X, Yang J, Ding H, LeBrun D, Ding K, Houchen CW, Postier RG, Ambrose CG, Li Z, Bi X, Li M. ZIP4 silencing improves bone loss in pancreatic cancer. Oncotarget. 2015;6:26041–26051. doi: 10.18632/oncotarget.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JA, Lee JK, Lee SY. Serum trace elements during treatment in pancreatic cancer patients and their associations with cancer prognosis. Clin Nutr. 2024;43:1459–1472. doi: 10.1016/j.clnu.2024.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 71.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 72.Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 73.Skrajnowska D, Bobrowska-Korczak B. Role of Zinc in immune system and anti-cancer defense mechanisms. Nutrients. 2019;11:2273. doi: 10.3390/nu11102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prasad AS, Bao B. Molecular mechanisms of Zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants (Basel) 2019;8:164. doi: 10.3390/antiox8060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst. 2005;97:1458–1465. doi: 10.1093/jnci/dji292. [DOI] [PubMed] [Google Scholar]
- 76.Xu X, Guo HJ, Xie HY, Li J, Zhuang RZ, Ling Q, Zhou L, Wei XY, Liu ZK, Ding SM, Chen KJ, Xu ZY, Zheng SS. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J Biol Sci. 2014;10:245–256. doi: 10.7150/ijbs.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan Q, Zhang W, Emerson RE, Xu Y. ZIP4 is a novel cancer stem cell marker in high-grade serous ovarian cancer. Cancers (Basel) 2020;12:3692. doi: 10.3390/cancers12123692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Q, Li L, Wang TL, Emerson RE, Xu Y. A novel ZIP4-HDAC4-VEGFA axis in high-grade serous ovarian cancer. Cancers (Basel) 2021;13:3821. doi: 10.3390/cancers13153821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, Cui X, Chen L, Yan W, Jiang T, Hergenroeder GW, Fletcher SA, Levine JM, Kim DH, Tandon N, Zhu JJ, Li M. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 2013;15:1008–1016. doi: 10.1093/neuonc/not042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li M, Fan K, Zheng B, Zekria D, Suo T, Liu H, Shen S, Liu H, Ni X. Knockdown of SLC39A4 expression inhibits the proliferation and motility of gallbladder cancer cells and tumor formation in nude mice. Cancer Manag Res. 2021;13:2235–2246. doi: 10.2147/CMAR.S282269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng Q, Liu YM, Liu J, Han J, Guo JX, Lu S, Huang XM, Yi P, Lang JY, Zhang P, Wang CT. Inhibition of ZIP4 reverses epithelial-to-mesenchymal transition and enhances the radiosensitivity in human nasopharyngeal carcinoma cells. Cell Death Dis. 2019;10:588. doi: 10.1038/s41419-019-1807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Y, Zhan H, Zhang Y, Yang J, Liu M, Xu C, Fan X, Zhang J, Zhou Z, Shi X, Ramesh R, Li M. ZIP4 promotes non-small cell lung cancer metastasis by activating snail-N-cadherin signaling axis. Cancer Lett. 2021;521:71–81. doi: 10.1016/j.canlet.2021.08.025. [DOI] [PubMed] [Google Scholar]
- 83.Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol. 2012;30:906–911. doi: 10.1016/j.urolonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Vogel-González M, Musa-Afaneh D, Rivera Gil P, Vicente R. Zinc favors triple-negative breast cancer’s microenvironment modulation and cell plasticity. Int J Mol Sci. 2021;22:9188. doi: 10.3390/ijms22179188. [DOI] [PMC free article] [PubMed] [Google Scholar]