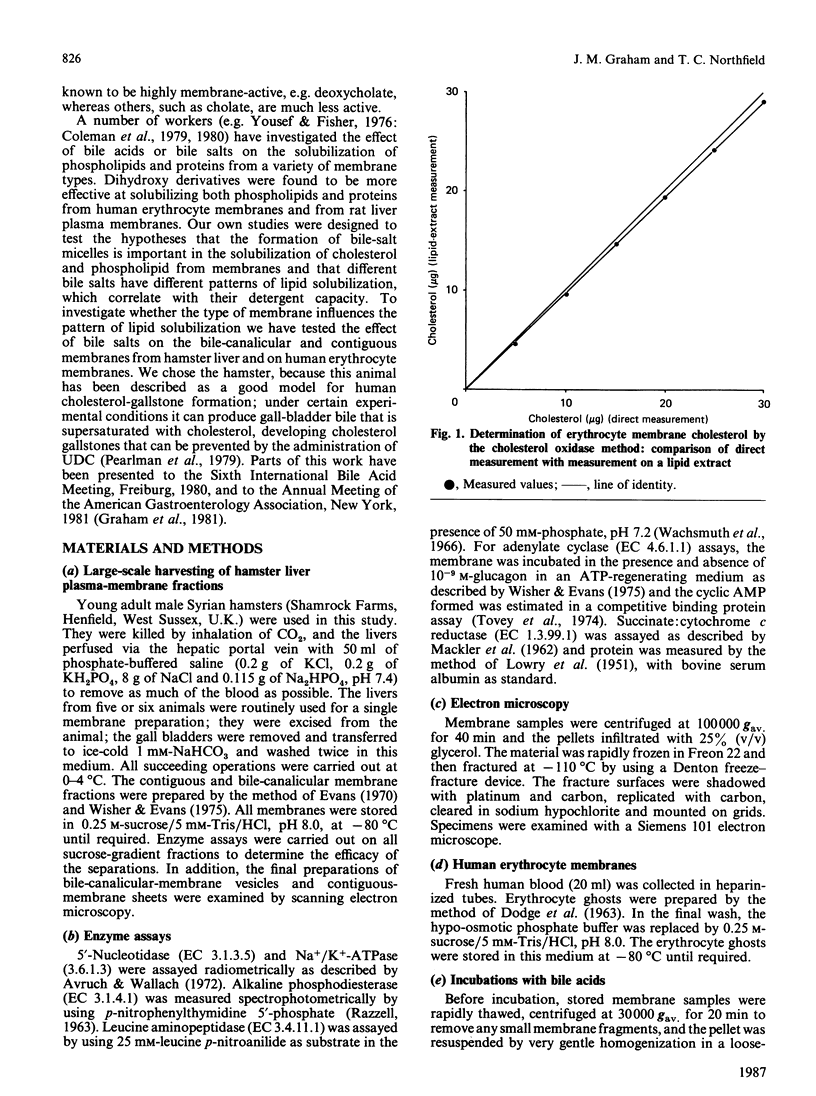
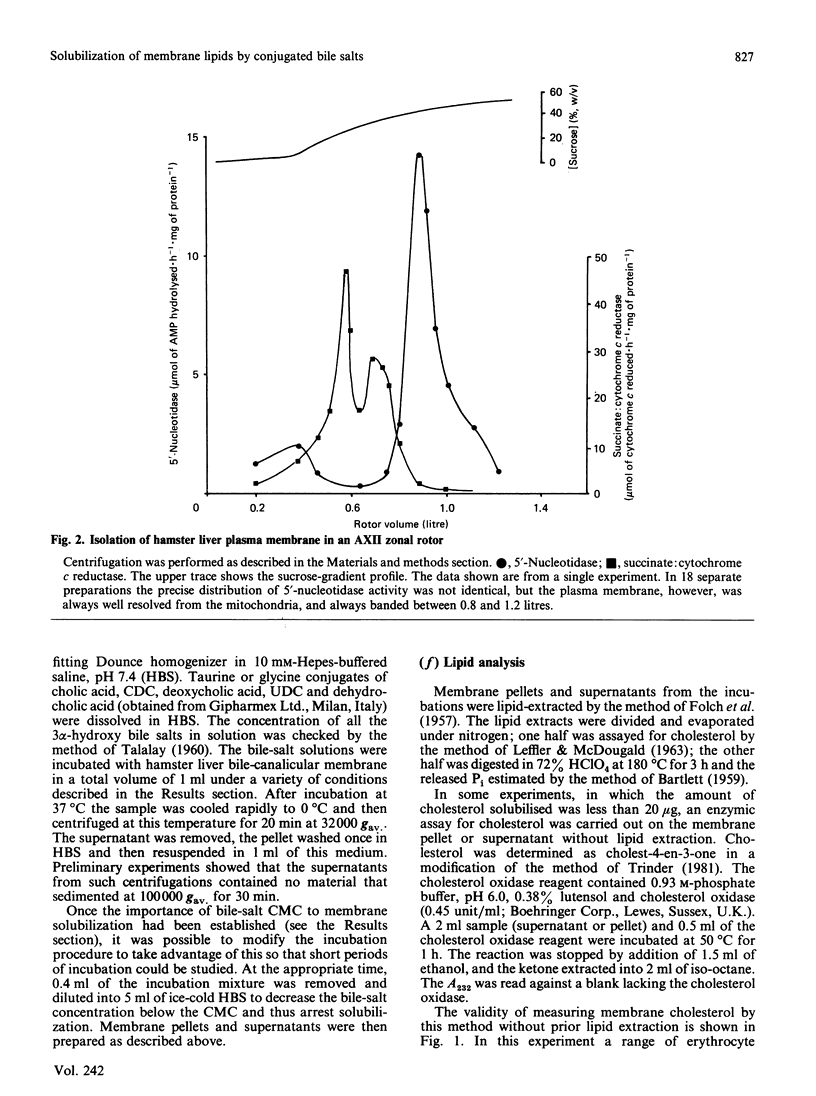
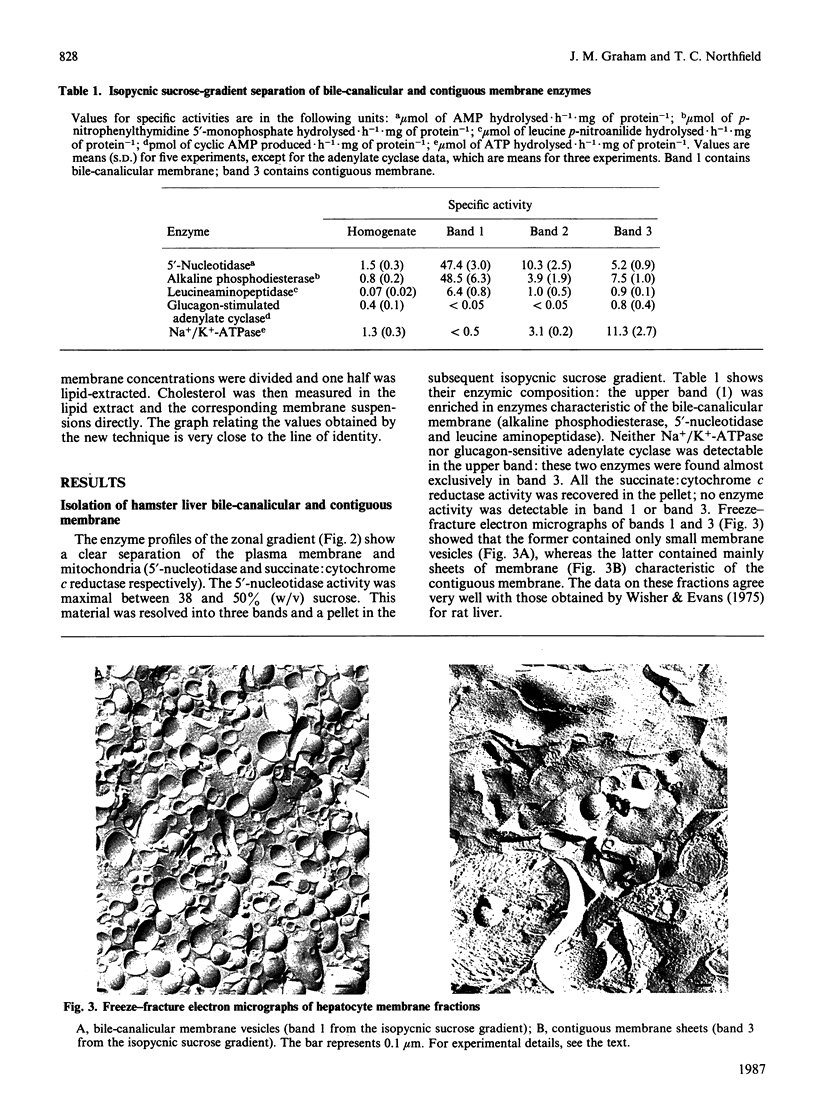
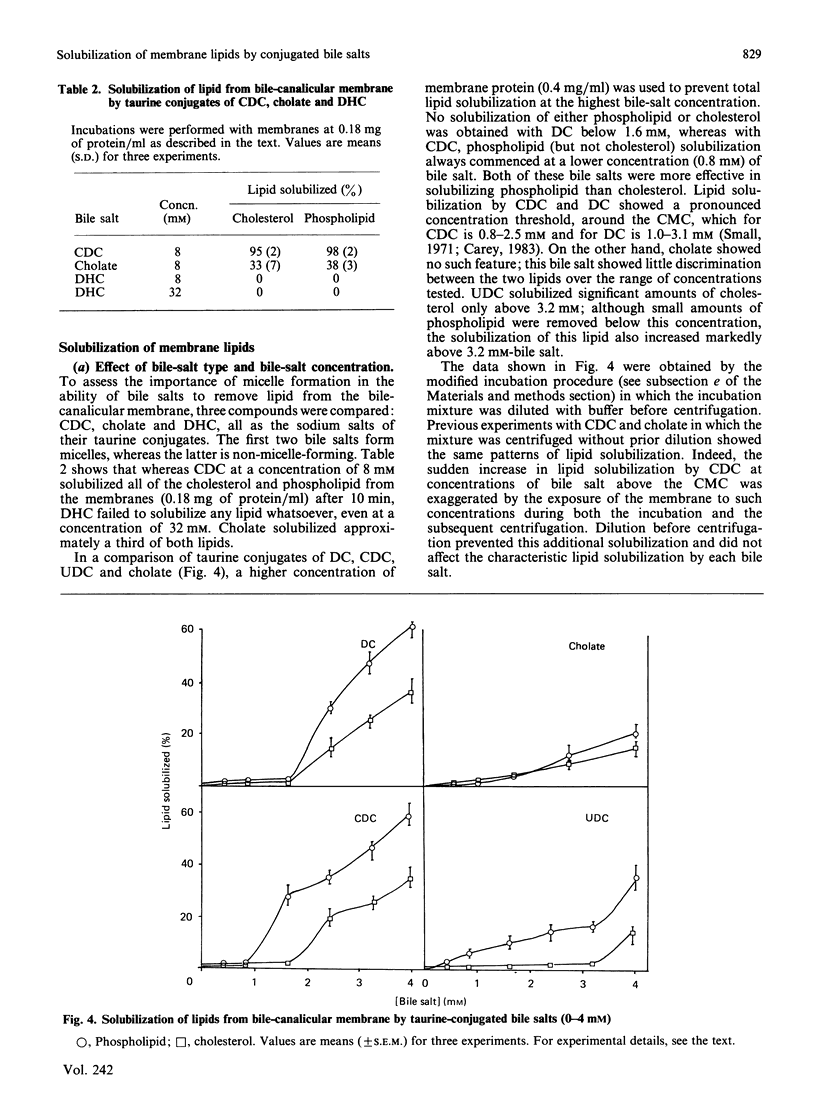
Abstract
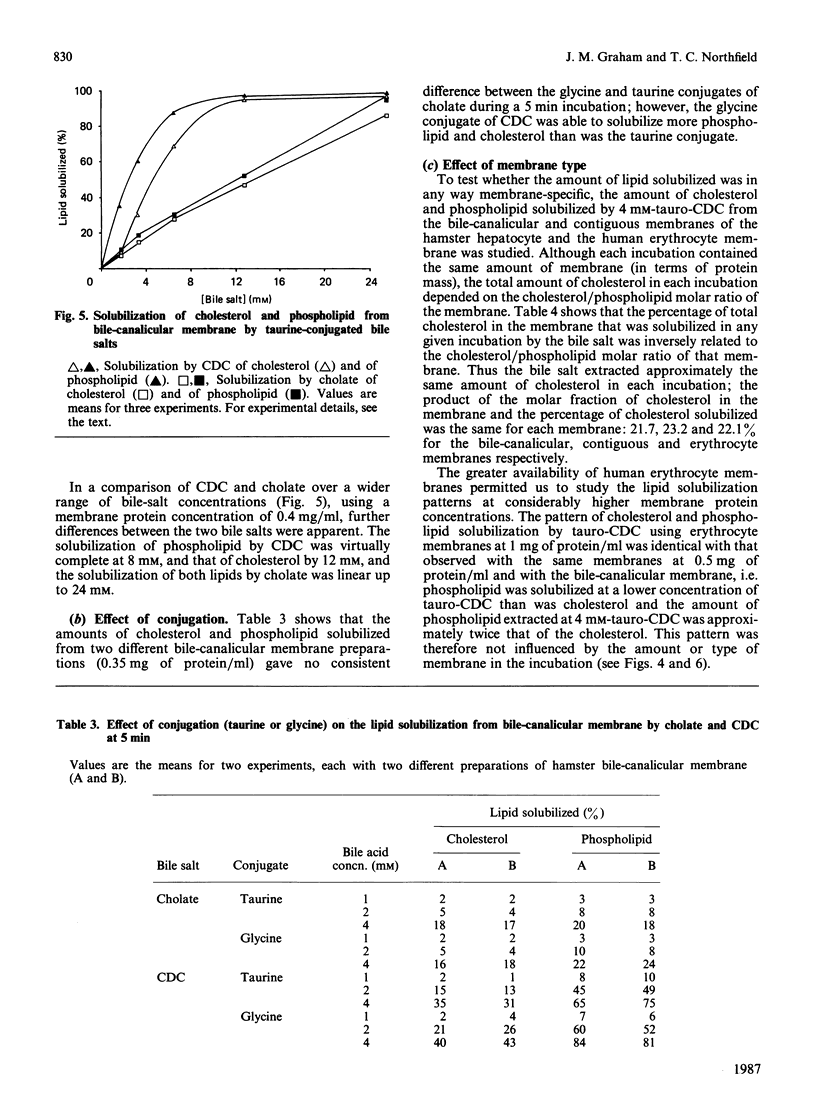
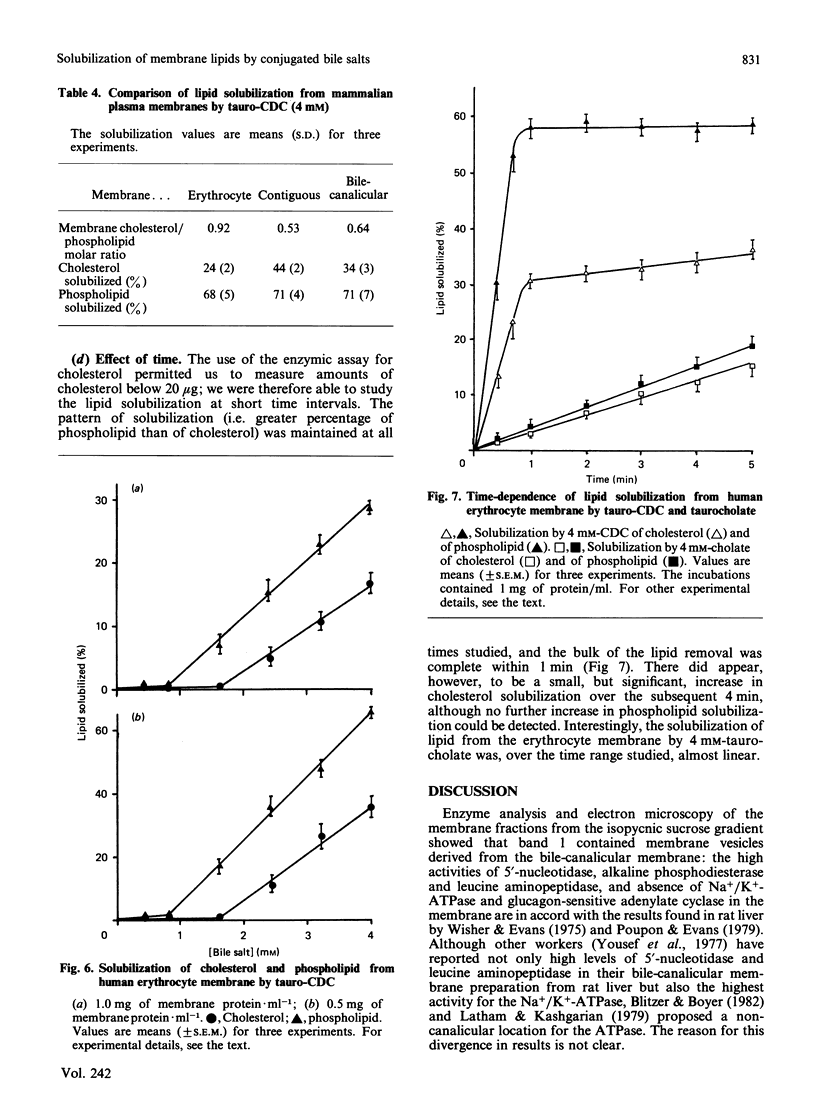
We have demonstrated in vitro the efficacy of the taurine-conjugated dihydroxy bile salts deoxycholate and chenodeoxycholate in solubilizing both cholesterol and phospholipid from hamster liver bile-canalicular and contiguous membranes and from human erythrocyte membrane. On the other hand, the dihydroxy bile salt ursodeoxycholate and the trihydroxy bile salt cholate solubilize much less lipid. The lipid solubilization by the four bile salts correlated well with their hydrophobicity: glycochenodeoxycolate, which is more hydrophobic than the tauro derivative, also solubilized more lipid. All the dihydroxy bile salts have a threshold concentration above which lipid solubilization increases rapidly; this correlates approximately with the critical micellar concentration. The non-micelle-forming bile salt dehydrocholate solubilized no lipid at all up to 32 mM. All the dihydroxy bile acids are much more efficient at solubilizing phospholipid than cholesterol. Cholate does not show such a pronounced discrimination. Lipid solubilization by chenodeoxycholate was essentially complete within 1 min, whereas that by cholate was linear up to 5 min. Maximal lipid solubilization with chenodeoxycholate occurred at 8-12 mM; solubilization by cholate was linear up to 32 mM. Ursodeoxycholate was the only dihydroxy bile salt which was able to solubilize phospholipid (although not cholesterol) below the critical micellar concentration. This similarity between cholate and ursodeoxycholate may reflect their ability to form a more extensive liquid-crystal system. Membrane specificity was demonstrated only inasmuch as the lower the cholesterol/phospholipid ratio in the membrane, the greater the fractional solubilization of cholesterol by bile salts, i.e. the total amount of cholesterol solubilized depended only on the bile-salt concentration. On the other hand, the total amount of phospholipid solubilized decreased with increasing cholesterol/phospholipid ratio in the membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accatino L., Simon F. R. Identification and characterization of a bile acid receptor in isolated liver surface membranes. J Clin Invest. 1976 Feb;57(2):496–508. doi: 10.1172/JCI108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barnwell S. G., Lowe P. J., Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984 Jun 15;220(3):723–731. doi: 10.1042/bj2200723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. D., Whitney B., Dowling R. H. Gallstone dissolution in man using chenodeoxycholic acid. Lancet. 1972 Dec 9;2(7789):1213–1216. doi: 10.1016/s0140-6736(72)92266-0. [DOI] [PubMed] [Google Scholar]
- Blitzer B. L., Boyer J. L. Cellular mechanisms of bile formation. Gastroenterology. 1982 Feb;82(2):346–357. [PubMed] [Google Scholar]
- Carey M. C., Montet J. C., Phillips M. C., Armstrong M. J., Mazer N. A. Thermodynamic and molecular basis for dissimilar cholesterol-solubilizing capacities by micellar solutions of bile salts: cases of sodium chenodeoxycholate and sodium ursodeoxycholate and their glycine and taurine conjugates. Biochemistry. 1981 Jun 9;20(12):3637–3648. doi: 10.1021/bi00515a052. [DOI] [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The characteristics of mixed micellar solutions with particular reference to bile. Am J Med. 1970 Nov;49:590–608. doi: 10.1016/s0002-9343(70)80127-9. [DOI] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Lowe P. J., Billington D. Membrane lipid composition and susceptibility to bile salt damage. Biochim Biophys Acta. 1980 Jun 20;599(1):294–300. doi: 10.1016/0005-2736(80)90075-9. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Danzinger R. G., Hofmann A. F., Schoenfield L. J., Thistle J. L. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972 Jan 6;286(1):1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Grundy S. M. Effects of feeding cholic acid and chenodeoxycholic acid on cholesterol absorption and hepatic secretion of biliary lipids in man. J Lipid Res. 1980 Jan;21(1):23–34. [PubMed] [Google Scholar]
- Evans W. H. Fractionation of liver plasma membranes prepared by zonal centrifugation. Biochem J. 1970 Mar;116(5):833–842. doi: 10.1042/bj1160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hardison W. G., Apter J. T. Micellar theory of biliary cholesterol excretion. Am J Physiol. 1972 Jan;222(1):61–67. doi: 10.1152/ajplegacy.1972.222.1.61. [DOI] [PubMed] [Google Scholar]
- Hegardt F. G., Dam H. The solubility of cholesterol in aqueous solutions of bile salts and lecithin. Z Ernahrungswiss. 1971 Apr;10(3):223–233. doi: 10.1007/BF02020933. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRusso N. F., Hoffman N. E., Hofmann A. F., Northfield T. C., Thistle J. L. Effect of primary bile acid ingestion on bile acid metabolism and biliary lipid secretion in gallstone patients. Gastroenterology. 1975 Dec;69(6):1301–1314. [PubMed] [Google Scholar]
- Latham P. S., Kashgarian M. The ultrastructural localization of transport ATPase in the rat liver at non-bile canalicular plasma membranes. Gastroenterology. 1979 May;76(5 Pt 1):988–996. [PubMed] [Google Scholar]
- Makino I., Shinozaki K., Yoshino K., Nakagawa S. [Dissolution of cholesterol gallstones by long-term administration of ursodeoxycholic acid]. Nihon Shokakibyo Gakkai Zasshi. 1975 Jun;72(6):690–702. [PubMed] [Google Scholar]
- Maton P. N., Murphy G. M., Dowling R. H. Ursodeoxycholic acid treatment of gallstones. Dose-response study and possible mechanism of action. Lancet. 1977 Dec 24;2(8052-8053):1297–1301. doi: 10.1016/s0140-6736(77)90358-0. [DOI] [PubMed] [Google Scholar]
- O'Máille E. R. The influence of micelle formation on bile salt secretion. J Physiol. 1980 May;302:107–120. doi: 10.1113/jphysiol.1980.sp013232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J. V., Payne A. W., Fitzharris B. M., Mullock B. M., Hinton R. H., Hall J. G. Comparative aspects of the hepatobiliary transport of IgA. Ann N Y Acad Sci. 1983 Jun 30;409:411–427. doi: 10.1111/j.1749-6632.1983.tb26886.x. [DOI] [PubMed] [Google Scholar]
- Pearlman B. J., Bonorris G. G., Phillips M. J., Chung A., Vimadalal S., Marks J. W., Schoenfield L. J. Cholesterol gallstone formation and prevention by chenodeoxycholic and ursodeoxycholic acids. A new hamster model. Gastroenterology. 1979 Oct;77(4 Pt 1):634–641. [PubMed] [Google Scholar]
- Poupon R. E., Evans W. H. Biochemical evidence that Na+,K+-ATPase is located at the lateral region of the hepatocyte surface membrane. FEBS Lett. 1979 Dec 15;108(2):374–378. doi: 10.1016/0014-5793(79)80567-0. [DOI] [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Small D. M. The formation of gallstones. Adv Intern Med. 1970;16:243–264. [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- Thistle J. L., Hofmann A. F. Efficacy and specificity of chenodeoxycholic acid therapy for dissolving gallstones. N Engl J Med. 1973 Sep 27;289(13):655–659. doi: 10.1056/NEJM197309272891303. [DOI] [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Trinder P. Oxidase determination of plasma cholesterol as cholest-4-en-3-one using iso-octane extraction. Ann Clin Biochem. 1981 Mar;18(Pt 2):64–70. doi: 10.1177/000456328101800202. [DOI] [PubMed] [Google Scholar]
- Vyvoda O. S., Coleman R., Holdsworth G. Effects of different bile salts upon the composition and morphology of a liver plasma membrane preparation. Deoxycholate is more membrane damaging than cholate and its conjugates. Biochim Biophys Acta. 1977 Feb 14;465(1):68–76. doi: 10.1016/0005-2736(77)90356-x. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Fritze I., Pfleiderer G. An aminopeptidase occurring in pig kidney. I. An improved method of preparation. Physical and enzymic properties. Biochemistry. 1966 Jan;5(1):169–174. doi: 10.1021/bi00865a022. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef I. M., Fisher M. M. In vitro effect of free bile acids on the bile canalicular membrane phospholipids in the rat. Can J Biochem. 1976 Dec;54(12):1040–1046. doi: 10.1139/o76-152. [DOI] [PubMed] [Google Scholar]
- Yousef I. M., Fisher M. M., Piekarski J., Holub B. J. Activity of phospholipid-synthesizing enzymes in rat liver plasma membranes and the source of biliary lecithin. Lipids. 1977 Feb;12(2):140–144. doi: 10.1007/BF02533283. [DOI] [PubMed] [Google Scholar]



