Abstract
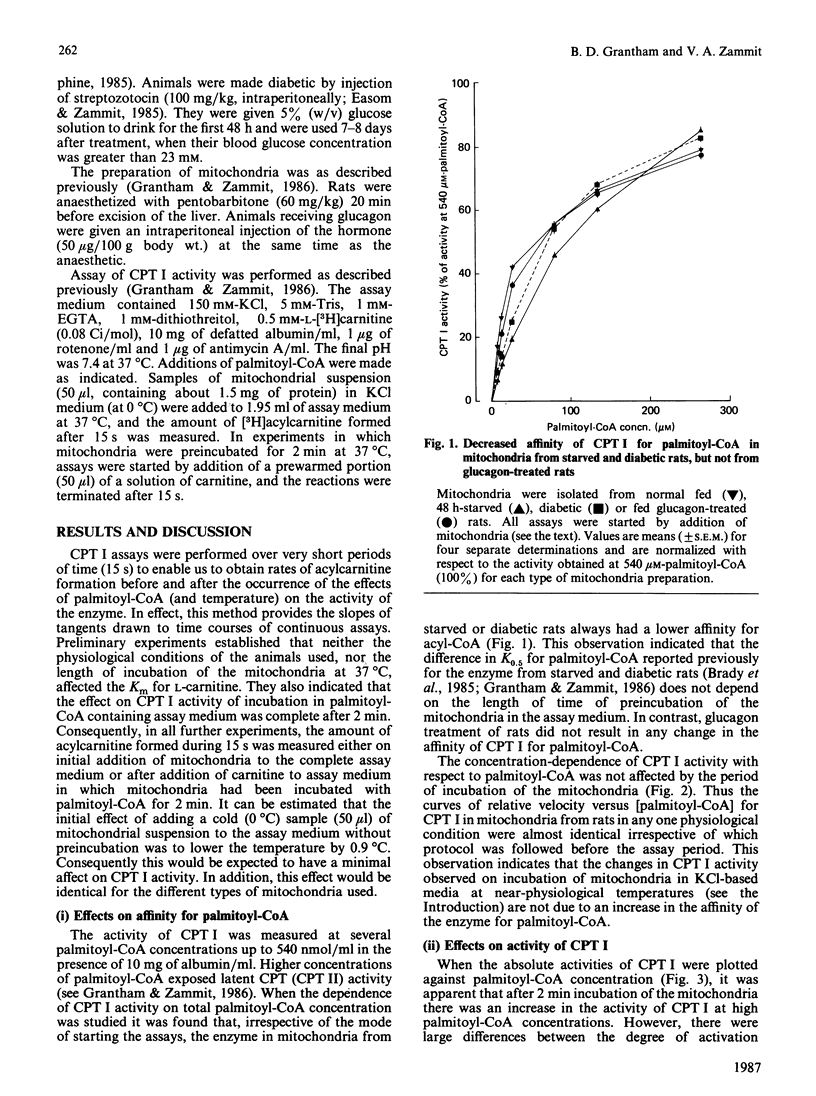
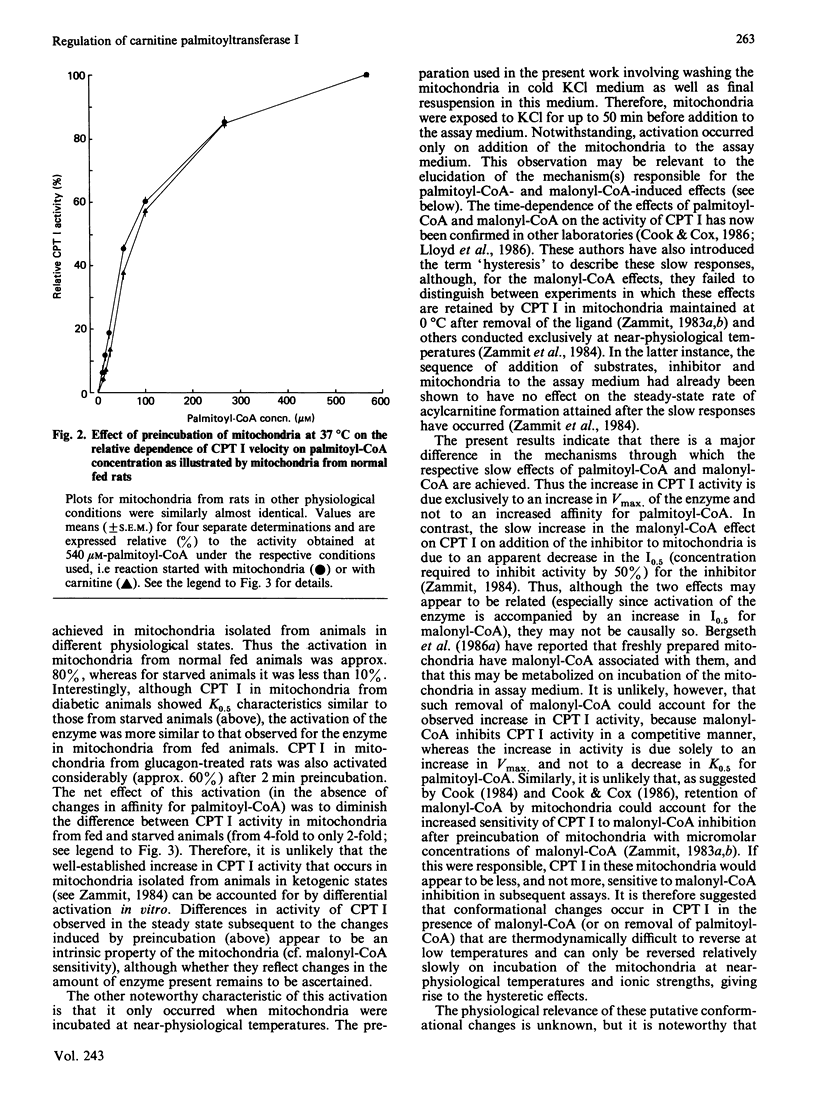
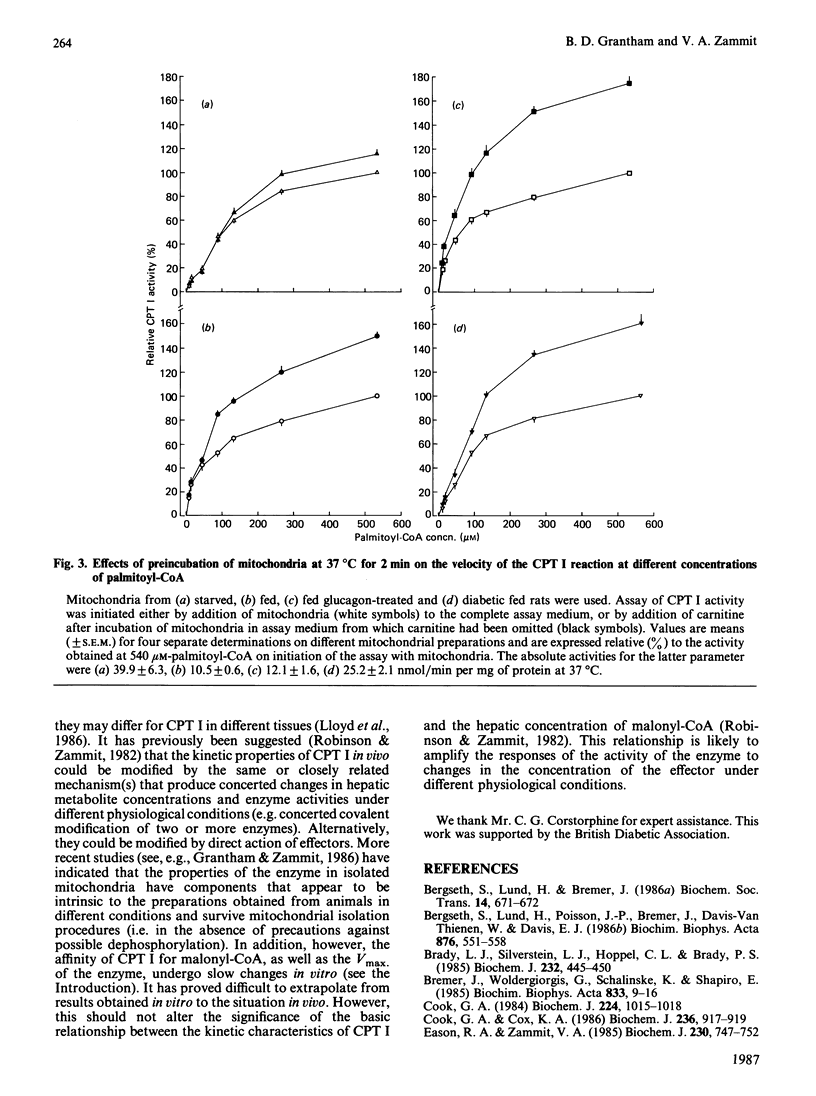
The activation of overt carnitine palmitoyltransferase activity that occurs when rat liver mitochondria are incubated at near-physiological temperatures and ionic strengths was studied for mitochondria obtained from animals in different physiological states. In all instances, it was found to be due exclusively to an increase in the catalytic capacity of the enzyme and not to an increase in affinity of the enzyme for palmitoyl-CoA. The enzyme in mitochondria from fed animals always showed a larger degree of activation than that in mitochondria from starved animals. This was the case even for mitochondria (e.g. from fed diabetic animals) in which the kinetic characteristics of carnitine palmitoyltransferase were more similar to those for the enzyme in mitochondria from starved rats. Glucagon treatment of rats before isolation of the mitochondria did not affect the characteristics either of the kinetic parameters of overt carnitine palmitoyltransferase or of its activation in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergseth S., Lund H., Bremer J. Is carnitine palmitoyltransferase inhibited by a malonyl-CoA-binding unit in the mitochondria? Biochem Soc Trans. 1986 Aug;14(4):671–672. doi: 10.1042/bst0140671. [DOI] [PubMed] [Google Scholar]
- Bergseth S., Lund H., Poisson J. P., Bremer J., Davis-Van Thienen W., Davis E. J. Carnitine palmitoyltransferase: activation and inactivation in liver mitochondria from fed, fasted, hypo- and hyperthyroid rats. Biochim Biophys Acta. 1986 May 21;876(3):551–558. doi: 10.1016/0005-2760(86)90043-3. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Silverstein L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner membrane properties and carnitine palmitoyltransferase A and B. Effect of diabetes and starvation. Biochem J. 1985 Dec 1;232(2):445–450. doi: 10.1042/bj2320445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Woldegiorgis G., Schalinske K., Shrago E. Carnitine palmitoyltransferase. Activation by palmitoyl-CoA and inactivation by malonyl-CoA. Biochim Biophys Acta. 1985 Jan 9;833(1):9–16. doi: 10.1016/0005-2760(85)90247-4. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Cox K. A. Hysteretic behaviour of carnitine palmitoyltransferase. The effect of preincubation with malonyl-CoA. Biochem J. 1986 Jun 15;236(3):917–919. doi: 10.1042/bj2360917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Involvement of hysteretic effects in the inhibition of carnitine palmitoyltransferase by malonyl-CoA. Biochem J. 1984 Dec 15;224(3):1015–1018. doi: 10.1042/bj2241015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. Effects of diabetes on the expressed and total activities of 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver in vivo. Reversal by insulin treatment. Biochem J. 1985 Sep 15;230(3):747–752. doi: 10.1042/bj2300747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. C., Carpenter C. A., Saggerson E. D. Intertissue differences in the hysteretic behaviour of carnitine palmitoyltransferase in the presence of malonyl-CoA. Biochem J. 1986 Jul 1;237(1):289–291. doi: 10.1042/bj2370289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Altered release of carnitine palmitoyltransferase activity by digitonin from liver mitochondria of rats in different physiological states. Biochem J. 1985 Sep 1;230(2):389–394. doi: 10.1042/bj2300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Gray S. R. Changes in the ability of malonyl-CoA to inhibit carnitine palmitoyltransferase I activity and to bind to rat liver mitochondria during incubation in vitro. Differences in binding at 0 degree C and 37 degrees C with a fixed concentration of malonyl-CoA. Biochem J. 1984 Sep 1;222(2):335–342. doi: 10.1042/bj2220335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Increased sensitivity of carnitine palmitoyltransferase I activity to malonyl-CoA inhibition after preincubation of intact rat liver mitochondria with micromolar concentrations of malonyl-CoA in vitro. Biochem J. 1983 Mar 15;210(3):953–956. doi: 10.1042/bj2100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Reversible sensitization and desensitization of carnitine palmitoyltransferase I to inhibition by malonyl-CoA in isolated rat liver mitochondria. Significance for the mechanism of malonyl-CoA-induced sensitization. Biochem J. 1983 Sep 15;214(3):1027–1030. doi: 10.1042/bj2141027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


