Abstract
The synthesis of S-adenosylmethionine (AdoMet) decarboxylase was studied by translating the rat prostate mRNA for this enzyme in a reticulocyte lysate. The protein was formed as a precursor of Mr 37,000, which was converted into the enzyme subunit of Mr 32,000 in the lysates. The presence of putrescine had no effect on the synthesis of the precursor of AdoMet decarboxylase, but accelerated its conversion into the enzyme subunit. Spermidine, spermine, decarboxylated AdoMet, AdoMet and methylglyoxal bis(guanylhydrazone) were not able to substitute for putrescine in this effect. These results indicate that, in addition to its direct activation of mammalian AdoMet decarboxylase, putrescine could increase the amount of the enzyme by increasing its production.
Full text
PDF
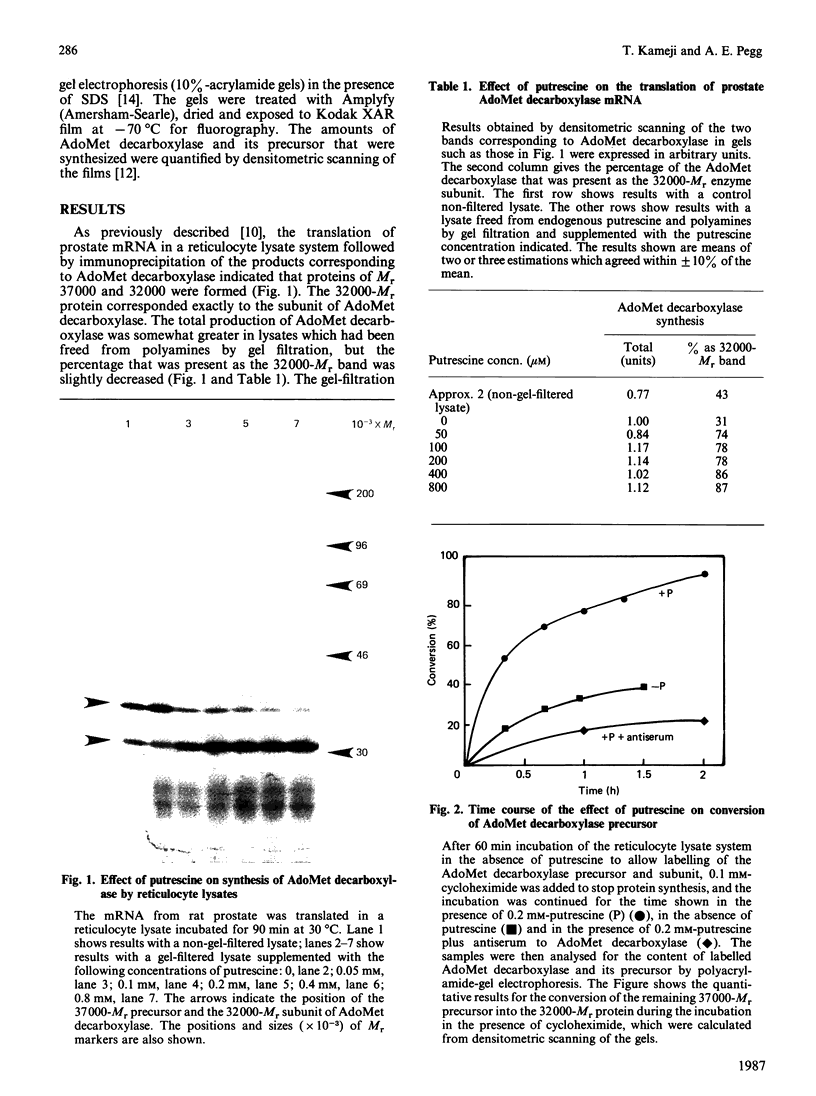


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. S-adenosylmethionine decarboxylase: a brief review. Cell Biochem Funct. 1984 Jan;2(1):11–15. doi: 10.1002/cbf.290020105. [DOI] [PubMed] [Google Scholar]
- Recsei P. A., Huynh Q. K., Snell E. E. Conversion of prohistidine decarboxylase to histidine decarboxylase: peptide chain cleavage by nonhydrolytic serinolysis. Proc Natl Acad Sci U S A. 1983 Feb;80(4):973–977. doi: 10.1073/pnas.80.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P. A., Snell E. E. Pyruvoyl enzymes. Annu Rev Biochem. 1984;53:357–387. doi: 10.1146/annurev.bi.53.070184.002041. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Christman K. L., Pegg A. E. Quantitation of S-adenosylmethionine decarboxylase protein. Biochemistry. 1985 Jul 30;24(16):4417–4423. doi: 10.1021/bi00337a024. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Increased content of mRNA for a precursor of S-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J Biol Chem. 1986 Oct 15;261(29):13833–13837. [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]