Abstract
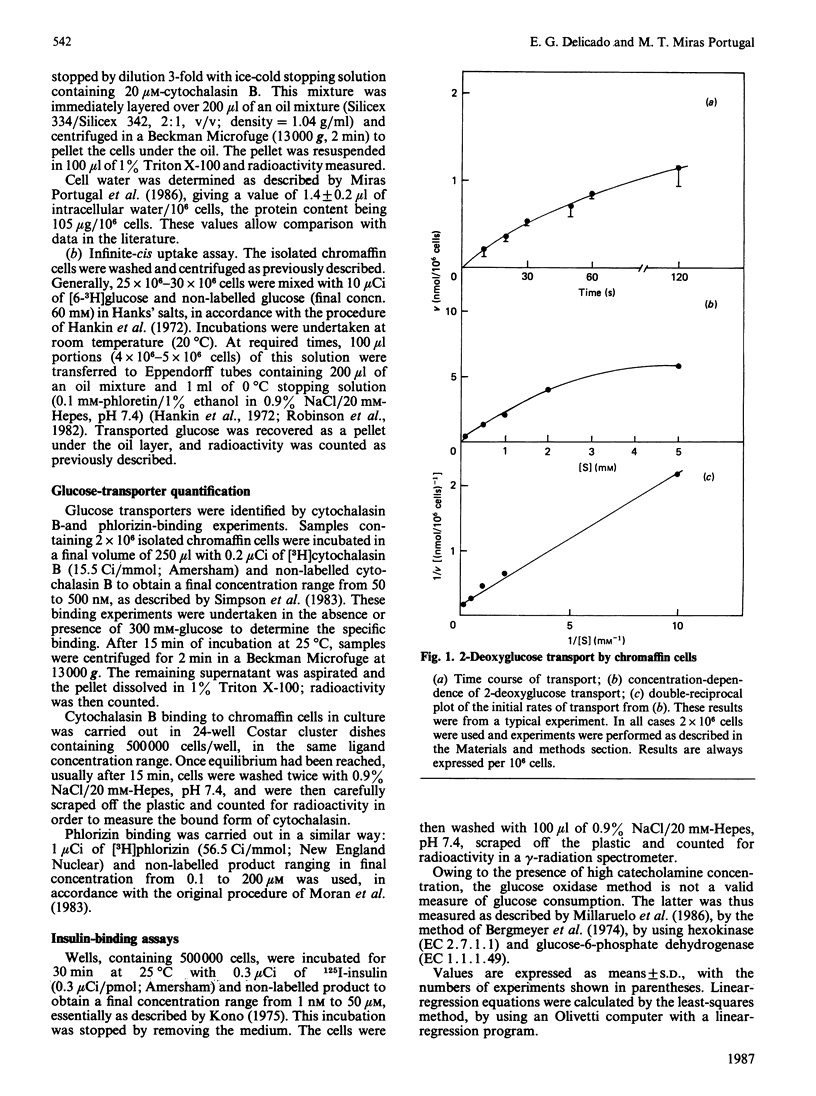
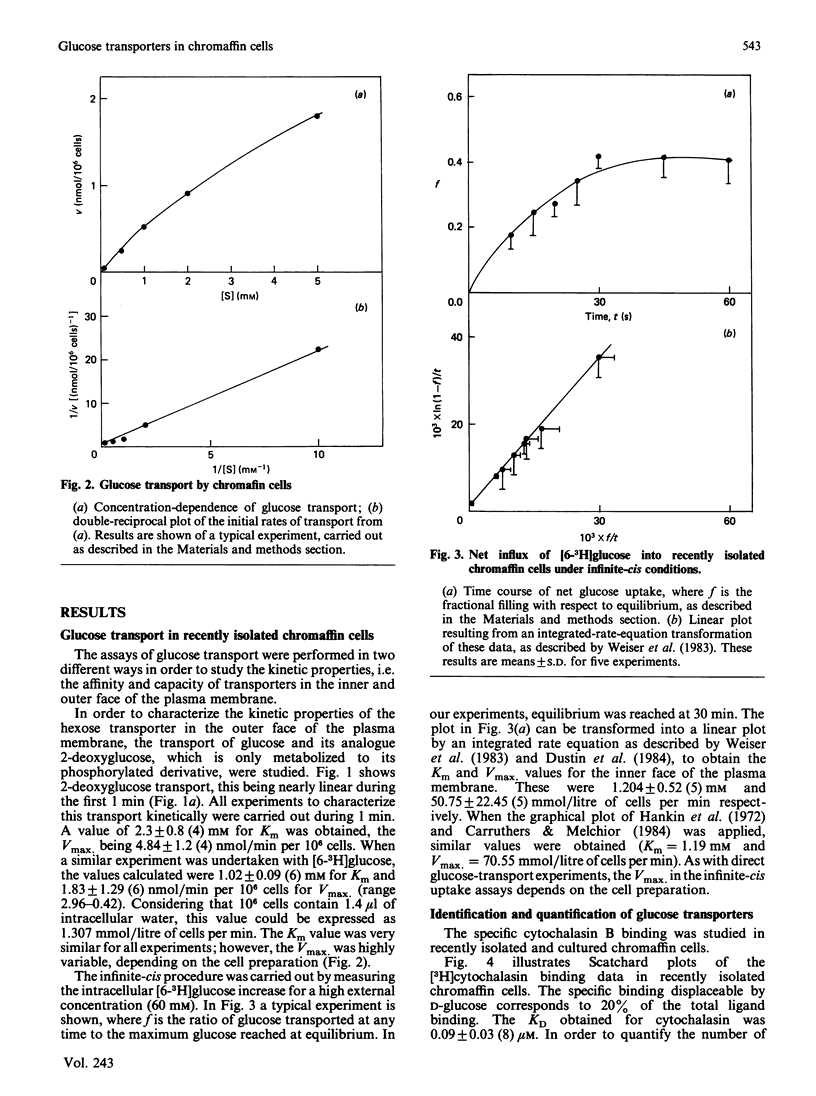
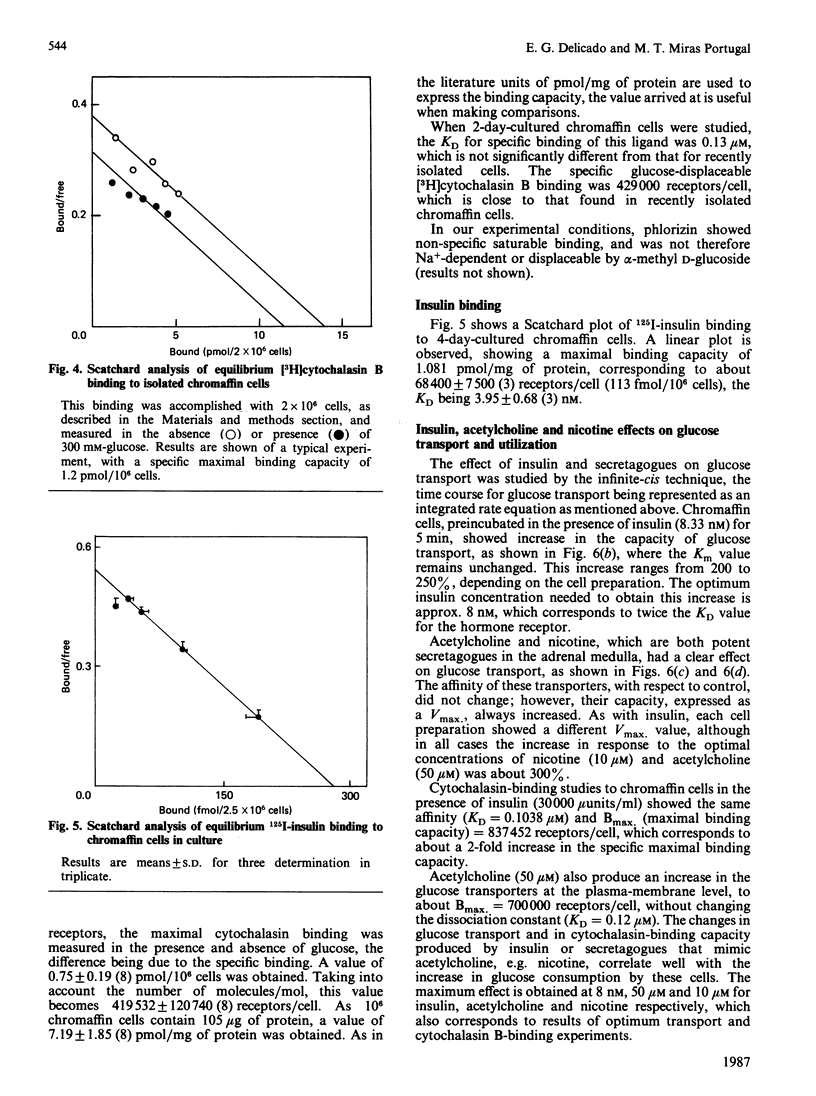
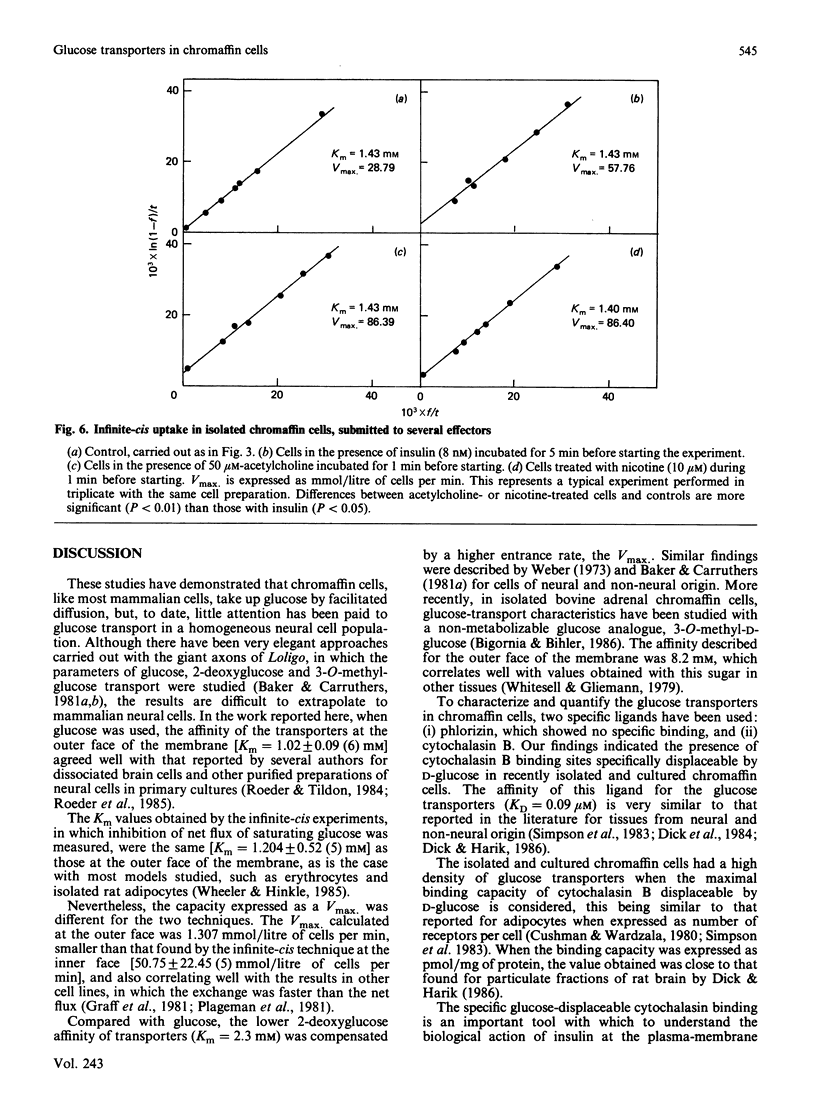
1. Isolated chromaffin cells from bovine adrenal medulla were used to study glucose transport in a homogeneous neural tissue. 2. The affinity of glucose transporters was 1.20 +/- 0.52 mM by the infinite-cis technique and 1.02 +/- 0.09 mM by the direct transport experiments. 3. The affinity for 2-deoxyglucose of these transporters was 2.3 mM. 4. The glucose transporters, quantified by [3H]cytochalasin B binding, were 419,532 +/- 120,740 receptors/cell, which corresponds to about 7.2 +/- 2 pmol/mg of protein, with KD = 0.1 microM. 5. High-affinity insulin receptors with KD = 3.95 nM were present at a density of 68,400 +/- 7500 per cell. 6. Insulin and secretagogues increased glucose transport, raising the transporter number at the plasma membrane without changes in the affinity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Carruthers A. 3-O-methylglucose transport in internally dialysed giant axons of Loligo. J Physiol. 1981 Jul;316:503–525. doi: 10.1113/jphysiol.1981.sp013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Sugar transport in giant axons of Loligo. J Physiol. 1981 Jul;316:481–502. doi: 10.1113/jphysiol.1981.sp013802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigornia L., Bihler I. 3-O-methyl-D-glucose uptake in isolated bovine adrenal chromaffin cells. Biochim Biophys Acta. 1986 Mar 14;885(3):335–344. doi: 10.1016/0167-4889(86)90249-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984 Jun 25;779(2):201–216. doi: 10.1016/0304-4157(84)90009-1. [DOI] [PubMed] [Google Scholar]
- Carruthers A., Melchior D. L. A rapid method of reconstituting human erythrocyte sugar transport proteins. Biochemistry. 1984 Jun 5;23(12):2712–2718. doi: 10.1021/bi00307a027. [DOI] [PubMed] [Google Scholar]
- Cech J. M., Freeman R. B., Jr, Caro J. F., Amatruda J. M. Insulin action and binding in isolated hepatocytes from fasted, streptozotocin-diabetic, and older, spontaneously obese rats. Biochem J. 1980 Jun 15;188(3):839–845. doi: 10.1042/bj1880839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Cynober L., Aussel C., Chatelain P., Vaubourdolle M., Agneray J., Ekindjian O. G. Insulin-like growth factor I/somatomedin C action on 2-deoxyglucose and alpha-amino isobutyrate uptake in chick embryo fibroblasts. Biochimie. 1985 Oct-Nov;67(10-11):1185–1190. doi: 10.1016/s0300-9084(85)80118-8. [DOI] [PubMed] [Google Scholar]
- Decker S., Lipmann F. Transport of D-glucose by membrane vesicles from normal and avian sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5358–5361. doi: 10.1073/pnas.78.9.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delicado E., Torres M., Miras-Portugal M. T. Effects of insulin on glucose transporters and metabolic patterns in Harding-Passey melanoma cells. Cancer Res. 1986 Aug;46(8):3762–3767. [PubMed] [Google Scholar]
- Dick A. P., Harik S. I. Distribution of the glucose transporter in the mammalian brain. J Neurochem. 1986 May;46(5):1406–1411. doi: 10.1111/j.1471-4159.1986.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I., Klip A., Walker D. M. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Jacobson G. R., Peterson S. W. Effects of insulin receptor down-regulation on hexose transport in human erythrocytes. J Biol Chem. 1984 Nov 25;259(22):13660–13663. [PubMed] [Google Scholar]
- Flint D. J., Sinnett-Smith P. A., Clegg R. A., Vernon R. G. Role of insulin receptors in the changing metabolism of adipose tissue during pregnancy and lactation in the rat. Biochem J. 1979 Aug 15;182(2):421–427. doi: 10.1042/bj1820421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura L. M., Barnea E. R., Biggers W., Naftolin F., Sanyal M. K. Insulin modulates neuronal plasma membrane development in human fetal spinal cord neurons in culture. Neurosci Lett. 1986 Apr 24;65(3):283–286. doi: 10.1016/0304-3940(86)90275-2. [DOI] [PubMed] [Google Scholar]
- Graff J. C., Wohlhueter R. M., Plagemann P. G. Hexose transport in Novikoff rat hepatoma cells. A simple carrier with directional symmetry, but variable relative mobilities of loaded and empty carrier. Biochim Biophys Acta. 1981 Mar 6;641(2):320–333. doi: 10.1016/0005-2736(81)90489-2. [DOI] [PubMed] [Google Scholar]
- Hankin B. L., Lieb W. R., Stein W. D. Rejection criteria for the asymmetric carrier and their application to glucose transport in the human red blood cell. Biochim Biophys Acta. 1972 Oct 23;288(1):114–126. doi: 10.1016/0005-2736(72)90229-5. [DOI] [PubMed] [Google Scholar]
- Hendricks S. A., de Pablo F., Roth J. Early development and tissue-specific patterns of insulin binding in chick embryo. Endocrinology. 1984 Oct;115(4):1315–1323. doi: 10.1210/endo-115-4-1315. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Des Rosiers M. H., Jehle J. W., Reivich M., Sharpe F., Sokoloff L. Mapping of functional neural pathways by autoradiographic survey of local metabolic rate with (14C)deoxyglucose. Science. 1975 Mar 7;187(4179):850–853. doi: 10.1126/science.1114332. [DOI] [PubMed] [Google Scholar]
- Kono T. Determination of the binding characteristics of insulin to fat cells. Methods Enzymol. 1975;37:193–198. doi: 10.1016/s0076-6879(75)37014-6. [DOI] [PubMed] [Google Scholar]
- Millaruelo A. I., Sagarra M. R., Delicado E., Torres M., Miras-Portugal M. T. Enzymes and pathways of glucose utilization in bovine adrenal medulla. Mol Cell Biochem. 1986 Apr;70(1):67–76. doi: 10.1007/BF00233804. [DOI] [PubMed] [Google Scholar]
- Millaruelo A., Sagarra M. R., Miras-Portugal M. T. Glycogen metabolism in bovine adrenal medulla. J Neurochem. 1982 Feb;38(2):470–476. doi: 10.1111/j.1471-4159.1982.tb08652.x. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal M. T., Torres M., Rotllan P., Aunis D. Adenosine transport in bovine chromaffin cells in culture. J Biol Chem. 1986 Feb 5;261(4):1712–1719. [PubMed] [Google Scholar]
- Moran A., Turner R. J., Handler J. S. Regulation of sodium-coupled glucose transport by glucose in a cultured epithelium. J Biol Chem. 1983 Dec 25;258(24):15087–15090. [PubMed] [Google Scholar]
- Perrin D., Aunis D. Reorganization of alpha-fodrin induced by stimulation in secretory cells. Nature. 1985 Jun 13;315(6020):589–592. doi: 10.1038/315589a0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Graff J., Erbe J., Wilkie P. Broad specificity hexose transport system with differential mobility of loaded and empty carrier, but directional symmetry, is common property of mammalian cell lines. J Biol Chem. 1981 Mar 25;256(6):2835–2842. [PubMed] [Google Scholar]
- Pollard H. B., Stopak S. S., Pazoles C. J., Creutz C. E. A simple and novel method for radiometric analysis of glucose utilization by adrenal chromaffin cells. Anal Biochem. 1981 Jan 15;110(2):424–430. doi: 10.1016/0003-2697(81)90214-1. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hendricks S. A., Quarum M., Roth J. The insulin receptor of rat brain is coupled to tyrosine kinase activity. J Biol Chem. 1984 Mar 25;259(6):3470–3474. [PubMed] [Google Scholar]
- Robinson F. W., Blevins T. L., Suzuki K., Kono T. An improved method of reconstitution of adipocyte glucose transport activity. Anal Biochem. 1982 May 1;122(1):10–19. doi: 10.1016/0003-2697(82)90244-5. [DOI] [PubMed] [Google Scholar]
- Roeder L. M., Williams I. B., Tildon J. T. Glucose transport in astrocytes: regulation by thyroid hormone. J Neurochem. 1985 Nov;45(5):1653–1657. doi: 10.1111/j.1471-4159.1985.tb07239.x. [DOI] [PubMed] [Google Scholar]
- Rotllan P., Miras Portugal M. T. Adenosine kinase from bovine adrenal medulla. Eur J Biochem. 1985 Sep 2;151(2):365–371. doi: 10.1111/j.1432-1033.1985.tb09110.x. [DOI] [PubMed] [Google Scholar]
- Rotllán P., Miras-Portugal M. T. Purine nucleotide synthesis in adrenal chromaffin cells. J Neurochem. 1985 Apr;44(4):1029–1036. doi: 10.1111/j.1471-4159.1985.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1(1):7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Standaert M. L., Pollet R. J. Equilibrium model for insulin-induced receptor down-regulation. Regulation of insulin receptors in differentiated BC3H-1 myocytes. J Biol Chem. 1984 Feb 25;259(4):2346–2354. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Weiser M. B., Razin M., Stein W. D. Kinetic tests of models for sugar transport in human erythrocytes and a comparison of fresh and cold-stored cells. Biochim Biophys Acta. 1983 Jan 19;727(2):379–388. doi: 10.1016/0005-2736(83)90423-6. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Whitesell R. R., Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979 Jun 25;254(12):5276–5283. [PubMed] [Google Scholar]
- Yorek M. A., Spector A. A., Ginsberg B. H. Characterization of an insulin receptor in human Y79 retinoblastoma cells. J Neurochem. 1985 Nov;45(5):1590–1595. doi: 10.1111/j.1471-4159.1985.tb07231.x. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Goens M. B., Hanaway P. J., Vinych J. V. Characterization and regulation of insulin receptors in rat brain. J Neurochem. 1984 May;42(5):1354–1362. doi: 10.1111/j.1471-4159.1984.tb02795.x. [DOI] [PubMed] [Google Scholar]


