Abstract
Introduction
Aging declines executive functions, including attentional function and inhibitory control, which is the ability to inhibit inappropriate or irrelevant responses. Certain types of background music are negatively correlated with cognitive function. The prefrontal network is correlated with task performance related to executive function. This study aimed to assess the impact of listening to background music on inhibition control and prefrontal cortical (PFC) activation measured using functional near-infrared spectroscopy (fNIRS) in healthy older people.
Methods
In total, 59 healthy volunteers, including 32 healthy older and 27 younger individuals (mean age ± standard deviation: 69 ± 7 and 32 ± 8 years, respectively), participated in this study. The participants completed the inhibition control task (the go/no-go task) and a similar task while listening to certain melodies of children’s songs that are popular in Japan. Changes in cerebral blood flow in the PFC during each task were evaluated using multichannel fNIRS. The relative changes in oxygenated hemoglobin (oxy-Hb) levels during the no-go and go tasks under the music and no-music conditions were compared using a paired t-test. Among the channels with a significant difference in oxy-Hb levels during the go/no-go task between the music and no-music conditions in the older group, the correlation between changes in accuracy response and oxy-Hb levels was validated using Pearson’s correlation test.
Results
The task accuracy was significantly reduced under the music condition compared with that under the no-music condition in the older group but not in the younger group. The accuracy reduction was significantly greater in the older group than in the younger group. In older people, the oxy-Hb levels in 20 channels located in the bilateral Broadman area (BA) 9 and BA46 in the dorsolateral prefrontal cortex and the bilateral BA10 in the frontal pole cortex significantly increased during the no-go tasks under the music condition. During the go/no-go task under the music condition, the decline in task accuracy was significantly correlated with increased oxy-Hb levels in six channels located in the bilateral BA10 in older people.
Conclusion
Background music induced the decline of inhibition control and increase of PFC activity in healthy older adults.
Keywords: aging, background music, functional near-infrared spectroscopy, go/no-go, inhibition control, older adult, prefrontal cortical activation
Introduction
Aging leads to a decline in executive functions, including attentional function, inhibitory control, working memory, and dual-task performance [1,2]. In particular, inhibition control, which is the ability to inhibit inappropriate or irrelevant responses, is the core component of executive functions [1]. Because several daily activities are related to response inhibition, reduction in inhibition control may lead to accidents in daily life and impairments in the quality of life of individuals. The decline in the performance of executive function and attention tests, including the go/no-go test and Stroop test, has been reported to be associated with a risk of future falls after two and five years [3,4]. Therefore, it is important to assess the ability of inhibition control of older people. The go/no-go test, in which participants are required to make a response to specific target stimuli (go) but not to the other stimuli (no-go), is widely used to assess attention and response inhibition [5-7].
Currently, music is prevalent in the daily lives of numerous people. Some individuals occasionally listen to music while performing tasks such as driving, studying, cooking, and exercising. There have been both positive and negative reports regarding the effects of music on cognitive function. Some reports have shown that background music has positive effects on cognitive functions. Preferred background music enhances task-focused attentional state on an easy task [8]. Listening to music, which is relaxing, alleviates mental fatigue and reduces attentional control impairment in undergraduate students [5]. A previous report showed that acoustic background music has no detrimental effects on inhibitory function and neural activation, as assessed using the go/no-go task and event-related potentials [7].
However, some studies have shown that listening to background music is negatively associated with cognitive function, including attentional control, concentration, and working memory [9-13]. A previous meta-analysis revealed that background music, compared to no music, negatively affects cognitive functions including reading process and memory [14]. Furthermore, compared with silence, background music can impair visual associative memory performance in healthy older adults but not in younger ones [15]. Music in the operation theater distracts novice surgeons from performing new tasks [10]. Compared with provocative music or silence, some types of music (e.g., relaxing music) can interfere with attentional control [11]. Happy and high-valence background music is also associated with faster response times in selective attention and greater activations of frontal-parietal areas. Meanwhile, sad and low-valence background music is associated with slower responses and greater occipital recruitment [16].
Cerebral hemodynamic changes related to executive function have been investigated using neuroimaging studies. The prefrontal network, including the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex, and frontal pole (FP), is associated with task performance related to executive function and attention control, as determined using functional magnetic resonance imaging and positron emission tomography [17,18]. Recently, functional near-infrared spectroscopy (fNIRS), a noninvasive method for measuring prefrontal cortical (PFC) hemodynamic changes, has been used to investigate brain activation during various tasks, including attention control and inhibition control [19-21].
A previous study review revealed that older adults had greater activation or additional recruitment of PFC regions in cognitive task performance, including executive function, than younger ones [22]. Recent studies on the effects of aging on executive function related to driving have shown that older adults commit more errors and exhibit greater PFC activation than younger ones [23,24]. Therefore, they have additional activated brain circuits to compensate for executive functions reduced by aging [23,24]. However, some studies have shown no difference between younger and healthy older adults in terms of the effects of music on attention control performance and brain activation [11,16].
To the best of our knowledge, no studies have investigated the impact of background music on PFC hemodynamics using fNIRS during attention and inhibition control in healthy older adults. This study aimed to examine the impact of listening to background music on inhibition control using the go/no-go task and on PFC activation using fNIRS in older adults. We hypothesized that background music can reduce inhibition control and induce PFC activation in healthy older adults.
Materials and methods
Design, setting, and ethics approval
This experimental study was conducted at the Chubu Medical Center for Prolonged Traumatic Brain Dysfunction of Chubu Neurorehabilitation Hospital in Minokamo, Japan. It was approved by the Ethics Committee of Kizawa Memorial Hospital, which was renamed to Chubu Neurorehabilitation Hospital on January 1, 2022 (approval number: 2020-003), and was conducted in accordance with the principles of the Declaration of Helsinki.
Participants
Right-handed healthy volunteers (aged 20-40 years (the young group) and 60-80 years (the older group)) were recruited using a poster about the study in Chubu Medical Center for Prolonged Traumatic Brain Dysfunction, Minokamo, Japan. All participants provided written informed consent. The older group underwent the Frontal Assessment Battery, Logical Memory (LM) Ⅰ and II tests of the Wechsler Memory Scale−Revised (WMS-R), and clinical dementia scale (CDR) to confirm the healthy condition without dementia and mild cognitive impairment (MCI). The exclusion criteria were as follows: (1) people with dementia or suspected dementia based on the revised Hasegawa’s Dementia Scale (HDS−R) [25] and Mini-Mental State Examination (MMSE) [26] (<24 points) in older group; (2) people with mild cognitive impairment (MCI) and suspected MCI based on WMS-R LM II (≤8 for 16 years of education, ≤4 for 10−15 years, ≤2 for 0−9 years) and the clinical dementia scale (≥0.5) [27] in older group; (3) those with impaired hearing or color blindness in both groups; (4) those who could not push the button for the go/no-go task; and (5) those with a history of traumatic brain injury, stroke, and other central nervous system lesions in both groups.
Study protocol
The participants in both groups underwent the go/no-go task, which is the inhibition control task comprising instructions using color presentation under the no-music conditions (i.e., silence) and music conditions on the same day. The condition (with or without music) that should be used first was randomly selected using a random number table created by a computer. The interval between the two conditions was 10 minutes, during which the participants rested in the sitting position.
Behavioral data collection (go/no-go task)
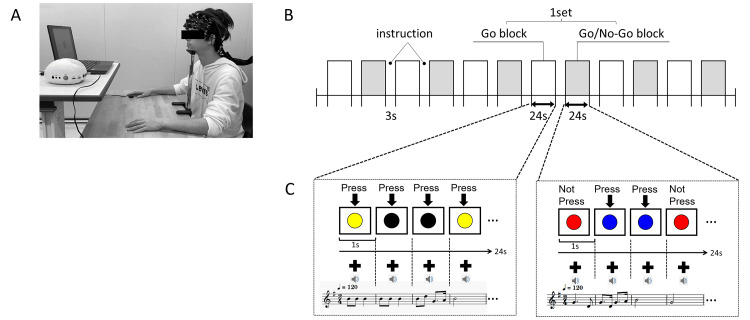
The inhibition control function was assessed using the go/no-go task. Based on previous fNIRS studies using the go/no-go task [6], the time course of the go/no-go task was decided. Figure 1 shows the procedure. The go/no-go task was performed using a laptop computer with high-resolution audio (LIFEBOOK, FUJITSU, Kanagawa, Japan) with the stimulus presentation software (PPT2BLUE, Shimazu Co., Kyoto, Japan) linked to the near-infrared spectroscopy (NIRS) system. The laptop monitor was placed approximately 70 cm in front of the participant’s head (Figure 1A).
Figure 1. (A) Image of the participants performing the go/no-go task and fNIRS measurements. (B) Experimental protocol. (C) Examples of colored circles that should be pressed or not and music.
fNIRS: functional near-infrared spectroscopy
The procedure comprised six block sets, including alternating go (baseline) block and go/no-go (target) blocks (Figure 1B). After three seconds of display for instructions, each block lasted for 24 seconds, with an overall block-set time of 54 seconds and a total session time of 5.5 minutes. In the go brock (go task), the participants were presented with a random sequence of two types of color circles, and they were instructed to press the button for both circles. In the go/no-go block (no-go task), the participants presented with no-go circles 50% of the time. Thus, they were required to respond to half of the trials (go trials) and not to respond to the other half (no-go trials). The color circles were presented at a frequency of 1 Hz during the go and go/no-go blocks. At the start of each block in the go task, the participants were instructed to press the display for the two colored circles (e.g., yellow and black). In contrast, in the no-go task, the participants were required to press a colored (e.g., blue) circle and not to press the other colored (e.g., red) circle (Figure 1C) on the display. There were three seconds between each block. Each participant underwent a practice block before any measurements to ensure their understanding of the instructions. Each participant was instructed to respond with the index finger of their dominant hand immediately and as correctly as possible according to the instructions when they saw the colored circle. The accurate response rate (%) was calculated as (correct response/total response numbers under the no-go tasks) × 100 in each participant.
Music condition
Under the music condition, the participants were instructed to listen to the background music carefully while undergoing the go/no-go task. The children’s songs used under the music condition were “I have been working on the Railroad,” “Jingle Bells,” “Picnic,” “Mickey Mouse Club March,” “Ah, Lovely Meadows,” and “Tenohira wo Taiyo ni (Put Your Hands up to the Sun),” which were selected from the children’s songbook [28] and were used as supplementary teaching materials in music classes in Japanese elementary schools. These songs were popular in Japan. However, the accurate familiarity among the participants was not quantified.
Melodies without lyrics of those songs were created with piano sounds for 24 measures using music editing software (Domio, TAKABO SOFT). All melodies were set to the 2/4-time signature, and a tempo of 120 beats per minute was used as one measure of one second. Each melody in the mp3 file was divided into one measure and attached to each slide to synchronize the playing of sounds, thereby resulting in the playback of 24 measures of melodies (Figure 1C). The order in which the six melodies were played was randomized for each go block and go/no-go block. The sound of the PC, which presented the color circle for the go/no-go task and the music, was set at a comfortable volume (approximately 50 dB), and the device was placed straightforward from the participants.
fNIRS
Changes in the cerebral blood flow in the prefrontal cortex during each task were observed using a 42-channel NIRS device with three wavelengths of near-infrared light (780, 805, and 380 nm) (SPEERDNIRS, Shimazu Co., Kyoto, Japan). The sampling rate was set at 8.3 Hz. The participants sat in a chair with their chin fixed on a desk, and the NIRS probes were set to cover the forehead and identify the prefrontal cortex. A 3 × 9 multichannel probe holder was used. The holder was attached to the lower anterior lines of the probe holder on the T3-Fpz-T4 line of the international 10-20 system. The source-detector distance was 3 cm. A high-pass filter uses cut-off frequencies of 0.01 Hz to remove baseline drift, and a 0.8-Hz low-pass filter removes heart-beat pulsations. The optical data were analyzed using the modified Beer-Lambert Law [6,29].
This equipment could measure the concentration of oxygenated hemoglobin (oxy-Hb), deoxygenated hemoglobin, and total hemoglobin. Changes in oxy-Hb level can better reflect cortical activity as it directly responds more to cognitive task-related brain activation and is more strongly correlated with blood oxygenation level-dependent signals measured on fMRI [30]. Hence, in this study, oxy-Hb level was used as the primary outcome measure.
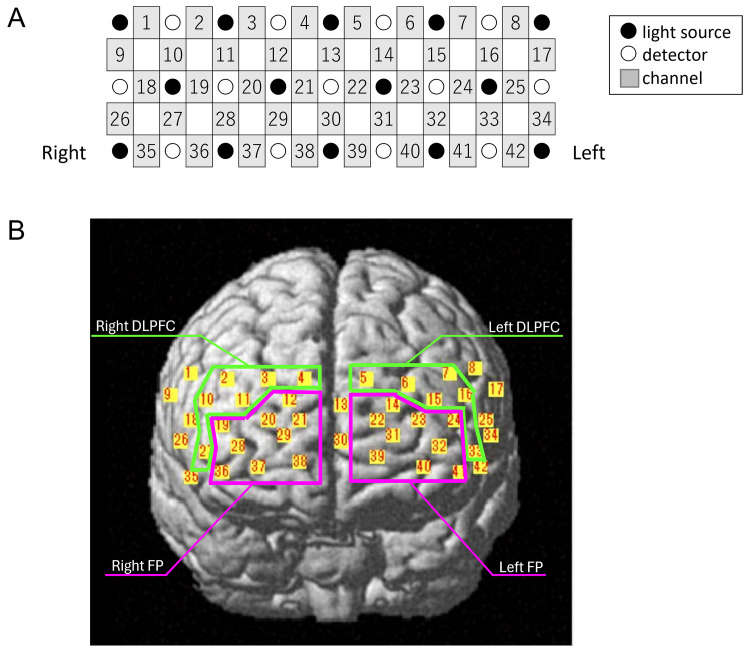
We determined the spatial values of the sourced and detector optode locations in each channel of the elastic cap using a three-dimensional digitizing pen [31]. Then, the Montreal Neurological Institute coordinate of each channel was detected using NIRS-SPM ver. 4, and the locations of the gyrus and Broadman area (BA) of each channel were identified using the WFU Pick Atlas ver. 3. The channels located in the DLPFC and FP cortex, which are associated with attention control, inhibition control, and dual-task performance [17-20], were used in the analysis.
The signal time course in each channel was calculated. The raw data in each channel among the individual participants were visually inspected. Moreover, we excluded the trial set with artifact signals, including discontinuous waveforms with a sudden large amplitude in both oxy-Hb and deoxygenated hemoglobin in the same direction by visual observation based on previous fNIRS studies [32,33]. Differences in the integrated values (average in seconds) of Hb signals of the target (average during 4-24 seconds after the go/no-go trial onset) and baseline (average during 0-10 seconds before the go/no-go trial onset (i.e., 14-24 seconds after the go trial onset)) periods in each channel were calculated and considered as oxy-Hb values involved in the no-go trial [6]. Finally, the changes in mean oxy-Hb levels during the no-go task under the music condition were compared with those under the no-music condition in each channel in the younger and older groups.
Statistical analysis
Shapiro-Wilk test was used to assess the distribution of age, accurate response rate, and neuropsychological test results. Data were presented as numbers and mean ± standard deviation or 95% confidence interval (95% CI) for variables with a normal distribution or median (first, third quartiles) for variables with a non-normal distribution.
The chi-square test was used to compare the ratio of participants between the two groups according to sex. Unpaired t-test or Mann-Whitney U test was used to compare the variables between the younger and older groups, as appropriate. A paired t-test or Wilcoxon signed-rank test was used to compare the accuracy response under the music and no-music conditions in the younger and older groups. To test for period and carry-over effects, the Mann-Whitney U test was used to examine the difference in and the sums of the summary scores for accuracy in the first and second sessions. One sample t-test against zero was used to assess significant changes in oxy-Hb levels during the no-go task against the go task in each channel under no-music and music conditions. A paired t-test was used to compare the oxy-Hb values under the music and no-music conditions in each group. Among the channels with a significant increase in oxy-Hb levels during the go/no-go task under the music condition compared with those under the no-music condition in the older group, the Pearson’s correlation test was applied to validate the association between changes in oxy-Hb level and changes in accurate response rates during the go/no-go task.
Statistical analyses were conducted using the IBM SPSS Statistics for Windows, Version 27 (Released 2020; IBM Corp., Armonk, New York, USA). A p-value of <0.05 indicated a statistically significant difference. P-values were corrected according to the number of NIRS channels with the Bonferroni method when analyzing the difference in oxy-Hb level changes in each channel between the no-go task and the go task under the music and no-music conditions. Bonferroni correction was also used for the results of the Shapiro-Wilk test. The correction was not applied for the abovementioned correlation tests as these correlations were exploratory [34]. The strength of the findings in terms of differences in oxy-Hb level changes under the music and no-music conditions was determined by calculating the effect size (Cohen’s d ≥ 0.2, small effect; ≥ 0.5, medium effect; and ≥ 0.8, large effect). In previous reports, the following correlation coefficients were considered: 0-0.19 as very weak; 0.20-0.39 as weak; 0.40-0.59 as moderate; 0.60-0.79 as strong; and 0.80-1 as very strong [35,36].
Results
Characteristics of the participants
Seventy healthy adults (28 in the younger group and 42 in the older group) were enlisted. Among them, one younger individual and eight older individuals were excluded from the analysis due to the presence of motion artifacts during NIRS measurement and analytical difficulties. Two individuals in the older group were also excluded due to low scores of MMSE, HDS−R, and LM II. Finally, 27 participants with a mean age of 32 ± 8 years in the younger group and 32 participants with a mean age of 69 ± 7 years in the older group were analyzed. Table 1 shows the characteristics of the participants. The Shapiro-Wilk test revealed the normal distribution of age, LM-I, and LM-II and the non-normal distribution of the accurate response in the go/no-go test, MMSE score, HDS-R, and FAB. The ratio of participants according to sex did not significantly differ between the younger and older groups. The neuropsychological examination scores of the older group were within normal limits (Table 1).
Table 1. Participants’ characteristics.
CDR: clinical dementia rating; FAB: frontal assessment battery; HDS-R: Revised Hasegawa Dementia Scale; MMSE: Mini-Mental State Examination; WMS-R: Wechsler Memory Scale-Revised; LM I: logical memory I; LM II: logical memory II
Data are presented as the number of participants, the mean ± standard deviation, or median (first and third quartiles).
| Characteristic | Young | Older |
| n | 27 | 32 |
| Men, n | 13 | 13 |
| Women, n | 14 | 19 |
| Age, y | 32 ± 8 | 69 ± 7 |
| MMSE | - | 28.5 (29.0, 30.0) |
| HDS-R | - | 28.5 (29.0, 30.0) |
| FAB | - | 17 (16, 18) |
| WMS-R LM I | - | 10.0 ± 3.1 |
| WMS-R LM II | - | 9.5 ± 3.4 |
| CDR | - | 0 ± 0 |
Behavioral performance (accurate response rate of the go/no-go task)
Table 2 shows the accurate response rates during the go/no-go task in each condition between the younger and older groups. All errors were only observed during the go/no-go task. The older group presented with minimal but statistically significant reductions in the accurate response rate during the go/no-go task under the music condition in comparison to no-music conditions (median: 97.2% vs. 98.6%, p = 0.002). In contrast, there was no statistically significant difference in the accurate response rate in the younger group (median: 100% vs. 100%, p = 0.178). The decrease in task accuracy in the older group was statistically significant than that in the younger group (median: -1.4 vs. 0.0, p = 0.035). There were no significant period effects and carry-over effects in the healthy older adult group (p = 0.887 and 0.566, respectively) and in the younger adult group (p = 0.550 and 0.884, respectively).
Table 2. Accuracy response rate during the go/no-go task.
Data was shown as median (25, 75 percentile).
†p = 0.035 versus young group, *p = 0.002 versus no-music in the older group
| Group | No-music (%) | Music (%) | Difference |
| Younger | 100.0 (98.6, 100.0) | 100.0 (97.9, 100.0) | -0.0 (-0.7, 0.0) |
| Older | 98.6 (95.8, 100.0) | 97.2 (92.4, 98.6)* | -1.4 (-3.5, 0.0)† |
Changes in oxy-Hb levels during the no-go task against the go task under the music and no-music conditions in each group
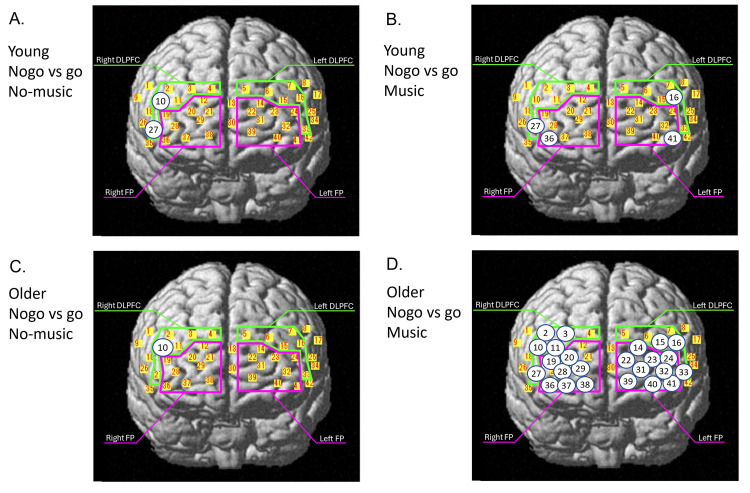
Thirty channels located in the bilateral DLPFC and FP were used in the NIRS analysis (Figure 2, Table 3). Tables 4, 5 show the oxy-Hb values between the no-go task and go task under no-music and music conditions in each channel in the younger adult (Table 4) and older adult groups (Table 5). The number of the channels, in which a significant increase in oxy-Hb levels during the no-go task against the go task was observed, increased from 2 in the right DLPFC (Ch10 and 27) under the no-music condition to 4, including 1 in the right DLPFC (Ch27), 1 in the left DLPFC (Ch16), 1 in the right FP (Ch36), and 1 in the left FP (Ch41), under the music condition in the younger adult group (Table 4, Figures 3A, 3B). Meanwhile, the number of channels increased from 1 in the right DLPFC (Ch10) to 24, including 5 in the right DLPFC (Ch2, Ch3, Ch10, Ch11, and Ch27), 3 in the left DLPFC (Ch15, Ch16, and Ch33), 7 in the right FP (Ch19, Ch20, Ch28, Ch29, Ch36, Ch37, and Ch38), and 9 in the left FP (Ch14, Ch22, Ch23, Ch24, Ch31, Ch32, Ch39, Ch40, and Ch41), in the older adult group (Table 5, Figures 3C, 3D).
Table 3. The location of each channel of NIRS.
BA: Broadman area; DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; MNI: Montreal Neurological Institute coordinates; NIRS: near-infrared spectroscopy
| Area | Side | Ch | MNI | BA | |||||
| X | Y | Z | |||||||
| mean | (SD) | mean | (SD) | mean | (SD) | ||||
| DLPFC | Right | 2 | 52 | (4) | 24 | (12) | 40 | (7) | BA9 |
| 3 | 36 | (5) | 44 | (9) | 40 | (8) | BA9 | ||
| 4 | 17 | (4) | 56 | (7) | 41 | (8) | BA9 | ||
| 10 | 59 | (4) | 19 | (12) | 29 | (5) | BA9 | ||
| 11 | 46 | (4) | 44 | (8) | 30 | (7) | BA46 | ||
| 27 | 59 | (3) | 32 | (9) | 8 | (5) | BA46 | ||
| Left | 5 | -6 | (5) | 56 | (6) | 42 | (7) | BA9 | |
| 6 | -26 | (6) | 49 | (8) | 41 | (6) | BA9 | ||
| 7 | -44 | (5) | 30 | (10) | 42 | (6) | BA9 | ||
| 15 | -38 | (4) | 48 | (7) | 31 | (5) | BA46 | ||
| 16 | -53 | (4) | 26 | (11) | 31 | (5) | BA9 | ||
| 33 | -54 | (3) | 36 | (8) | 8 | (4) | BA46 | ||
| FP | Right | 12 | 27 | (4) | 59 | (6) | 30 | (7) | BA10 |
| 19 | 53 | (4) | 40 | (8) | 20 | (6) | BA10 | ||
| 20 | 38 | (4) | 59 | (5) | 20 | (6) | BA10 | ||
| 21 | 18 | (3) | 69 | (3) | 22 | (6) | BA10 | ||
| 28 | 46 | (4) | 55 | (5) | 9 | (6) | BA10 | ||
| 29 | 28 | (4) | 69 | (2) | 11 | (6) | BA10 | ||
| 36 | 52 | (3) | 48 | (6) | -3 | (4) | BA10 | ||
| 37 | 38 | (4) | 65 | (3) | -1 | (5) | BA10 | ||
| 38 | 17 | (3) | 73 | (1) | 1 | (5) | BA10 | ||
| Left | 14 | -18 | (4) | 61 | (5) | 32 | (7) | BA10 | |
| 22 | -10 | (5) | 68 | (3) | 22 | (7) | BA10 | ||
| 23 | -30 | (5) | 62 | (5) | 21 | (5) | BA10 | ||
| 24 | -47 | (4) | 45 | (7) | 21 | (5) | BA10 | ||
| 31 | -21 | (4) | 70 | (2) | 12 | (5) | BA10 | ||
| 32 | -41 | (4) | 58 | (5) | 10 | (5) | BA10 | ||
| 39 | -13 | (3) | 73 | (1) | 2 | (4) | BA10 | ||
| 40 | -33 | (4) | 65 | (2) | 0 | (4) | BA10 | ||
| 41 | -48 | (3) | 50 | (5) | -2 | (3) | BA10 | ||
Table 4. Oxy-Hb values during go task and no-go task under no-music and music condition in younger group.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; SD: standard deviation; MD: mean difference; CI: confidence interval; p-value: p-value uncorrected; Sig: significance after Bonferroni correction; ns: not significant; R: right; L: left
| Area | Side | CH | No-music | Music | ||||||||||||
| Go (mM・cm) | Nogo (mM・cm) | MD (95% CI) | p-value | Sig | Go (mM・cm) | Nogo (mM・cm) | MD (95% CI) | p-value | Sig | |||||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||||||||
| DLPFC | R | 2 | 0.001 | 0.008 | 0.009 | 0.053 | 0.009 (-0.013, 0.03) | 0.413 | ns | -0.001 | 0.01 | 0.015 | 0.054 | 0.017 (-0.007, 0.04) | 0.152 | ns |
| 3 | 0.003 | 0.010 | 0.014 | 0.06 | 0.011 (-0.013, 0.035) | 0.340 | ns | 0.000 | 0.011 | 0.001 | 0.053 | 0.001 (-0.02, 0.023) | 0.918 | ns | ||
| 4 | 0.004 | 0.011 | 0.009 | 0.038 | 0.005 (-0.013, 0.022) | 0.588 | ns | 0.002 | 0.014 | 0.006 | 0.071 | 0.004 (-0.025, 0.033) | 0.786 | ns | ||
| 10 | -0.001 | 0.012 | 0.033 | 0.05 | 0.034 (0.016, 0.053) | 0.001 | sig | -0.003 | 0.008 | 0.025 | 0.057 | 0.028 (0.005, 0.051) | 0.019 | ns | ||
| 11 | 0.001 | 0.009 | 0.005 | 0.038 | 0.004 (-0.011, 0.019) | 0.563 | ns | -0.001 | 0.009 | 0.004 | 0.04 | 0.006 (-0.009, 0.02) | 0.453 | ns | ||
| 27 | -0.002 | 0.009 | 0.044 | 0.063 | 0.046 (0.022, 0.071) | 0.001 | sig | 0.000 | 0.008 | 0.035 | 0.051 | 0.036 (0.015, 0.056) | 0.001 | sig | ||
| L | 5 | 0.006 | 0.017 | 0.004 | 0.041 | -0.001 (-0.017, 0.014) | 0.845 | ns | 0.000 | 0.016 | 0.008 | 0.041 | 0.007 (-0.013, 0.027) | 0.463 | ns | |
| 6 | -0.002 | 0.009 | 0.013 | 0.072 | 0.015 (-0.021, 0.051) | 0.398 | ns | 0.000 | 0.017 | 0.017 | 0.087 | 0.017 (-0.032, 0.066) | 0.48 | ns | ||
| 7 | 0.003 | 0.010 | 0.016 | 0.073 | 0.013 (-0.016, 0.042) | 0.37 | ns | -0.003 | 0.011 | 0.009 | 0.069 | 0.013 (-0.018, 0.043) | 0.397 | ns | ||
| 15 | 0.000 | 0.013 | 0.010 | 0.083 | 0.01 (-0.021, 0.042) | 0.499 | ns | -0.001 | 0.011 | 0.018 | 0.053 | 0.02 (-0.001, 0.04) | 0.063 | ns | ||
| 16 | 0.002 | 0.012 | 0.013 | 0.076 | 0.011 (-0.018, 0.04) | 0.443 | ns | 0.001 | 0.011 | 0.027 | 0.038 | 0.025 (0.011, 0.04) | 0.001 | sig | ||
| 33 | -0.001 | 0.008 | 0.030 | 0.076 | 0.031 (0.001, 0.06) | 0.043 | ns | 0.000 | 0.01 | 0.03 | 0.045 | 0.03 (0.012, 0.048) | 0.002 | ns | ||
| FP | R | 12 | 0.000 | 0.008 | 0.011 | 0.043 | 0.011 (-0.006, 0.027) | 0.196 | ns | 0.001 | 0.008 | 0.009 | 0.04 | 0.008 (-0.009, 0.024) | 0.350 | ns |
| 19 | -0.001 | 0.009 | 0.020 | 0.052 | 0.021 (0.002, 0.041) | 0.033 | ns | -0.002 | 0.01 | 0.012 | 0.042 | 0.014 (-0.003, 0.031) | 0.098 | ns | ||
| 20 | -0.001 | 0.010 | 0.010 | 0.044 | 0.011 (-0.007, 0.028) | 0.21 | ns | 0.000 | 0.01 | 0.009 | 0.038 | 0.008 (-0.006, 0.023) | 0.251 | ns | ||
| 21 | 0.000 | 0.010 | 0.011 | 0.045 | 0.011 (-0.007, 0.03) | 0.219 | ns | 0.000 | 0.008 | 0.009 | 0.032 | 0.009 (-0.004, 0.021) | 0.156 | ns | ||
| 28 | -0.001 | 0.012 | 0.015 | 0.057 | 0.016 (-0.007, 0.039) | 0.161 | ns | 0.000 | 0.013 | 0.024 | 0.051 | 0.025 (0.005, 0.044) | 0.018 | ns | ||
| 29 | 0.003 | 0.016 | 0.005 | 0.058 | 0.003 (-0.022, 0.027) | 0.822 | ns | 0.002 | 0.014 | 0.021 | 0.054 | 0.019 (-0.003, 0.04) | 0.085 | ns | ||
| 36 | -0.001 | 0.010 | 0.030 | 0.055 | 0.031 (0.009, 0.053) | 0.007 | ns | 0.001 | 0.008 | 0.047 | 0.044 | 0.046 (0.028, 0.063) | 0.000 | sig | ||
| 37 | 0.001 | 0.016 | 0.015 | 0.066 | 0.014 (-0.015, 0.044) | 0.328 | ns | 0.000 | 0.011 | 0.03 | 0.055 | 0.03 (0.008, 0.052) | 0.008 | ns | ||
| 38 | 0.002 | 0.012 | 0.006 | 0.062 | 0.004 (-0.022, 0.03) | 0.741 | ns | 0.000 | 0.008 | 0.017 | 0.044 | 0.017 (0, 0.035) | 0.052 | ns | ||
| L | 14 | 0.001 | 0.010 | 0.000 | 0.092 | -0.001 (-0.037, 0.035) | 0.957 | ns | 0.000 | 0.011 | 0.02 | 0.061 | 0.02 (-0.005, 0.044) | 0.118 | ns | |
| 22 | 0.001 | 0.008 | 0.005 | 0.042 | 0.004 (-0.014, 0.022) | 0.641 | ns | 0.002 | 0.007 | 0.002 | 0.039 | 0 (-0.014, 0.015) | 0.958 | ns | ||
| 23 | 0.001 | 0.009 | 0.016 | 0.070 | 0.015 (-0.013, 0.044) | 0.273 | ns | 0.002 | 0.01 | 0.007 | 0.054 | 0.005 (-0.016, 0.026) | 0.638 | ns | ||
| 24 | -0.001 | 0.010 | 0.016 | 0.085 | 0.017 (-0.016, 0.05) | 0.295 | ns | -0.001 | 0.011 | 0.013 | 0.033 | 0.014 (0, 0.028) | 0.050 | ns | ||
| 31 | 0.004 | 0.012 | 0.007 | 0.070 | 0.004 (-0.025, 0.032) | 0.803 | ns | 0.002 | 0.012 | 0.012 | 0.056 | 0.01 (-0.012, 0.032) | 0.374 | ns | ||
| 32 | -0.002 | 0.014 | 0.015 | 0.075 | 0.018 (-0.013, 0.048) | 0.242 | ns | 0.000 | 0.012 | 0.02 | 0.058 | 0.02 (-0.002, 0.042) | 0.076 | ns | ||
| 39 | 0.001 | 0.011 | 0.016 | 0.056 | 0.015 (-0.008, 0.038) | 0.182 | ns | 0.002 | 0.01 | 0.023 | 0.045 | 0.021 (0.003, 0.038) | 0.021 | ns | ||
| 40 | 0.000 | 0.013 | 0.016 | 0.072 | 0.017 (-0.012, 0.046) | 0.25 | ns | -0.001 | 0.013 | 0.029 | 0.062 | 0.031 (0.006, 0.055) | 0.017 | ns | ||
| 41 | -0.001 | 0.009 | 0.038 | 0.077 | 0.039 (0.009, 0.069) | 0.012 | ns | 0.000 | 0.009 | 0.043 | 0.055 | 0.043 (0.021, 0.065) | 0.000 | sig | ||
Table 5. Oxy-Hb values during go task and no-go task under no-music and music condition in healthy older group.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; SD: standard deviation; MD: mean difference; CI: confidence interval; p-value: p-value uncorrected; Sig: significance after Bonferroni correction; ns: not significant; R: right; L: left
| Area | Side | Ch | No-music | Music | ||||||||||||
| Go (mM・cm) | Nogo (mM・cm) | MD (95% CI) | p-value | Sig | Go (mM・cm) | Nogo (mM・cm) | MD (95% CI) | p-value | Sig | |||||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||||||||
| DLPFC | R | 2 | -0.001 | 0.009 | 0.008 | 0.042 | 0.009 (-0.006, 0.025) | 0.217 | ns | -0.002 | 0.008 | 0.029 | 0.036 | 0.031 (0.017, 0.045) | 0.000 | sig |
| 3 | 0.000 | 0.007 | -0.008 | 0.034 | -0.008 (-0.02, 0.005) | 0.213 | ns | -0.002 | 0.007 | 0.022 | 0.038 | 0.024 (0.011, 0.038) | 0.001 | sig | ||
| 4 | -0.002 | 0.007 | 0.010 | 0.031 | 0.012 (-0.001, 0.024) | 0.062 | ns | -0.002 | 0.006 | 0.009 | 0.036 | 0.011 (-0.002, 0.024) | 0.087 | ns | ||
| 10 | -0.003 | 0.012 | 0.027 | 0.046 | 0.031 (0.013, 0.048) | 0.001 | sig | -0.004 | 0.007 | 0.042 | 0.042 | 0.046 (0.03, 0.061) | 0.000 | sig | ||
| 11 | 0.000 | 0.009 | 0.002 | 0.051 | 0.002 (-0.017, 0.02) | 0.870 | ns | -0.003 | 0.008 | 0.04 | 0.051 | 0.043 (0.024, 0.062) | 0.000 | sig | ||
| 27 | -0.002 | 0.008 | 0.028 | 0.057 | 0.03 (0.009, 0.051) | 0.007 | ns | -0.006 | 0.008 | 0.05 | 0.052 | 0.056 (0.037, 0.075) | 0.000 | sig | ||
| L | 5 | -0.002 | 0.006 | -0.003 | 0.028 | -0.001 (-0.015, 0.012) | 0.832 | ns | -0.003 | 0.005 | 0.005 | 0.025 | 0.007 (-0.005, 0.02) | 0.219 | ns | |
| 6 | 0.000 | 0.009 | 0.000 | 0.049 | -0.001 (-0.018, 0.017) | 0.928 | ns | -0.003 | 0.007 | 0.023 | 0.046 | 0.026 (0.008, 0.043) | 0.005 | ns | ||
| 7 | -0.002 | 0.01 | 0.003 | 0.037 | 0.005 (-0.01, 0.019) | 0.506 | ns | -0.003 | 0.008 | 0.021 | 0.073 | 0.024 (-0.001, 0.05) | 0.057 | ns | ||
| 15 | 0.000 | 0.009 | 0.009 | 0.045 | 0.01 (-0.006, 0.025) | 0.215 | ns | -0.004 | 0.008 | 0.041 | 0.049 | 0.045 (0.027, 0.064) | 0.000 | sig | ||
| 16 | -0.001 | 0.01 | 0.003 | 0.041 | 0.004 (-0.01, 0.018) | 0.542 | ns | -0.003 | 0.011 | 0.041 | 0.05 | 0.044 (0.026, 0.062) | 0.000 | sig | ||
| 33 | -0.002 | 0.008 | 0.027 | 0.06 | 0.029 (0.006, 0.052) | 0.014 | ns | -0.003 | 0.007 | 0.043 | 0.042 | 0.046 (0.031, 0.061) | 0.000 | sig | ||
| FP | R | 12 | 0.000 | 0.006 | -0.003 | 0.035 | -0.003 (-0.015, 0.01) | 0.640 | ns | -0.002 | 0.006 | 0.022 | 0.043 | 0.024 (0.007, 0.041) | 0.007 | ns |
| 19 | -0.001 | 0.01 | 0.010 | 0.061 | 0.011 (-0.011, 0.033) | 0.296 | ns | -0.005 | 0.008 | 0.044 | 0.057 | 0.049 (0.027, 0.07) | 0.000 | sig | ||
| 20 | 0.000 | 0.009 | 0.007 | 0.044 | 0.006 (-0.01, 0.023) | 0.433 | ns | -0.003 | 0.007 | 0.034 | 0.046 | 0.037 (0.019, 0.055) | 0.000 | sig | ||
| 21 | 0.000 | 0.008 | 0.000 | 0.036 | -0.001 (-0.014, 0.012) | 0.930 | ns | -0.002 | 0.006 | 0.02 | 0.044 | 0.023 (0.006, 0.039) | 0.009 | ns | ||
| 28 | -0.001 | 0.014 | 0.015 | 0.069 | 0.015 (-0.01, 0.041) | 0.222 | ns | -0.006 | 0.011 | 0.052 | 0.054 | 0.057 (0.036, 0.078) | 0.000 | sig | ||
| 29 | 0.001 | 0.012 | 0.001 | 0.049 | 0 (-0.018, 0.018) | 0.975 | ns | -0.003 | 0.007 | 0.036 | 0.054 | 0.039 (0.019, 0.06) | 0.000 | sig | ||
| 36 | 0.000 | 0.009 | 0.018 | 0.051 | 0.019 (0, 0.038) | 0.055 | ns | -0.005 | 0.007 | 0.047 | 0.047 | 0.052 (0.035, 0.07) | 0.000 | sig | ||
| 37 | 0.000 | 0.011 | 0.001 | 0.052 | 0.001 (-0.019, 0.02) | 0.933 | ns | -0.004 | 0.011 | 0.057 | 0.057 | 0.062 (0.04, 0.084) | 0.000 | sig | ||
| 38 | 0.000 | 0.01 | -0.016 | 0.046 | -0.016 (-0.035, 0.003) | 0.094 | ns | -0.004 | 0.009 | 0.04 | 0.049 | 0.044 (0.025, 0.063) | 0.000 | sig | ||
| L | 14 | -0.001 | 0.009 | -0.002 | 0.044 | -0.001 (-0.017, 0.015) | 0.902 | ns | -0.001 | 0.006 | 0.038 | 0.051 | 0.039 (0.02, 0.058) | 0.000 | sig | |
| 22 | 0.000 | 0.008 | -0.004 | 0.036 | -0.004 (-0.017, 0.009) | 0.550 | ns | -0.002 | 0.007 | 0.025 | 0.039 | 0.027 (0.012, 0.042) | 0.001 | sig | ||
| 23 | -0.001 | 0.009 | 0.007 | 0.040 | 0.008 (-0.007, 0.022) | 0.283 | ns | -0.002 | 0.007 | 0.038 | 0.044 | 0.04 (0.024, 0.055) | 0.000 | sig | ||
| 24 | -0.001 | 0.009 | 0.005 | 0.035 | 0.006 (-0.007, 0.018) | 0.387 | ns | -0.003 | 0.008 | 0.033 | 0.038 | 0.036 (0.022, 0.05) | 0.000 | sig | ||
| 31 | 0.000 | 0.01 | 0.006 | 0.052 | 0.006 (-0.012, 0.025) | 0.497 | ns | -0.004 | 0.007 | 0.047 | 0.045 | 0.051 (0.035, 0.067) | 0.000 | sig | ||
| 32 | -0.002 | 0.013 | 0.015 | 0.044 | 0.017 (0.001, 0.033) | 0.041 | ns | -0.005 | 0.008 | 0.055 | 0.046 | 0.059 (0.043, 0.076) | 0.000 | sig | ||
| 39 | 0.000 | 0.009 | -0.010 | 0.042 | -0.01 (-0.027, 0.007) | 0.225 | ns | -0.004 | 0.01 | 0.043 | 0.047 | 0.047 (0.029, 0.065) | 0.000 | sig | ||
| 40 | 0.000 | 0.011 | 0.010 | 0.047 | 0.01 (-0.007, 0.027) | 0.252 | ns | -0.005 | 0.009 | 0.051 | 0.051 | 0.056 (0.038, 0.074) | 0.000 | sig | ||
| 41 | -0.001 | 0.01 | 0.027 | 0.049 | 0.028 (0.01, 0.047) | 0.004 | ns | -0.005 | 0.007 | 0.044 | 0.049 | 0.049 (0.032, 0.066) | 0.000 | sig | ||
Figure 2. Channel location on the cortical surface. (A) Schematic illustration of the location of each optode and channel. (B) NIRS channels and regions of interest.
FP: frontal pole; DLPFC: dorsolateral prefrontal cortex; NIRS: near-infrared spectroscopy
Figure 3. The location of the channels in which a significant difference was observed between the no-go and go tasks under no-music conditions (A) and music conditions (B) in young adults and under no-music conditions (C) and music conditions (D) in healthy older adults.
FP: frontal pole; DLPFC: dorsolateral prefrontal cortex
Changes in oxy-Hb levels during the go/no-go tasks between the music and no-music conditions
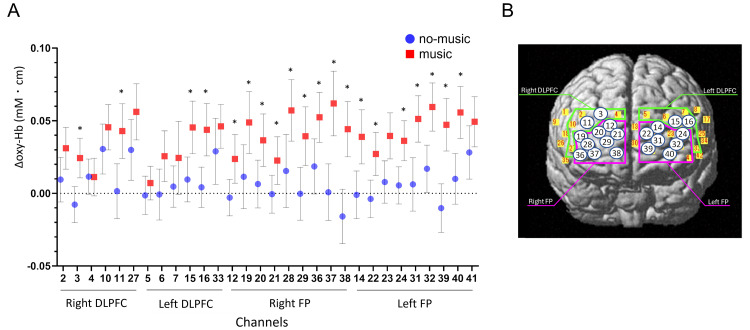
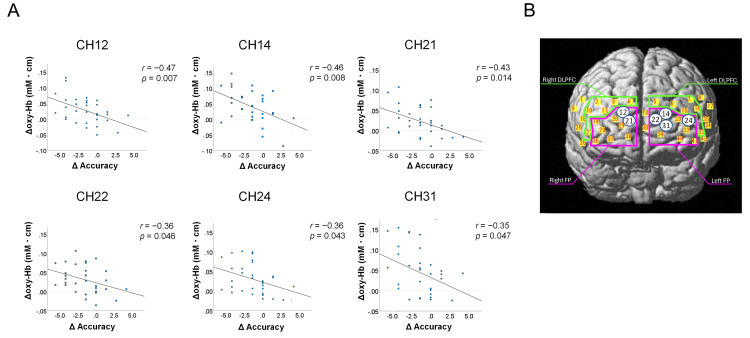
In the younger group, there were no significant differences in the increase in oxy-Hb levels between music and no-music conditions in all channels (Table 6). The increase in oxy-Hb levels in most channels in the older group was more likely to be greater under the music condition than under the no-music condition (Table 7). In the older group, moreover, the differences in oxy-Hb level changes in 20 channels located in the bilateral BA9 and BA46 (DLPFC) and the bilateral BA10 (FP) under the music and no-music conditions were statistically significant, and medium- or large-size effects were detected in these channels (Table 7, Figure 4). In six channels, including two channels in the right BA10 (Ch12 and 21) and four channels in the left BA10 (Ch14, 22, 24, and 31), the greater decline in the accurate response rate under the music condition was significantly correlated with a higher increase in oxy-Hb levels during the go/no-go task in the older group (Table 8, Figure 5). The correlation coefficient was 0.35-0.47, which indicated a weak to moderate correlation.
Table 6. Oxy-Hb changes during go/no-go task under music and no-music conditions in younger group.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; SD: standard deviation; MD: mean difference; CI: confidence interval; p-value: p-value uncorrected; Sig: significance after Bonferroni correction; ns: not significant; R: right; L: left
| Area | Side | CH | Music (mM・cm) (mean ± SD) | No-music (mM・cm) (mean ± SD) | MD (95% CI) | p-value | Sig | Cohen's d | Effect size |
| DLPFC | R | 2 | 0.017 ± 0.057 | 0.009 ± 0.054 | 0.008 (-0.022, 0.037) | 0.592 | ns | 0.14 | no |
| 3 | 0.001 ± 0.052 | 0.011 ± 0.058 | -0.010 (-0.037, 0.017) | 0.443 | ns | -0.19 | no | ||
| 4 | 0.004 ± 0.066 | 0.005 ± 0.04 | -0.001 (-0.03, 0.028) | 0.954 | ns | -0.02 | no | ||
| 10 | 0.028 ± 0.058 | 0.034 ± 0.047 | -0.006 (-0.034, 0.022) | 0.670 | ns | -0.11 | no | ||
| 11 | 0.006 ± 0.038 | 0.004 ± 0.037 | 0.001 (-0.021, 0.024) | 0.907 | ns | 0.04 | no | ||
| 27 | 0.036 ± 0.052 | 0.046 ± 0.062 | -0.011 (-0.042, 0.021) | 0.500 | ns | -0.18 | no | ||
| L | 5 | 0.007 ± 0.044 | -0.001 ± 0.034 | 0.009 (-0.012, 0.03) | 0.400 | ns | 0.22 | small | |
| 6 | 0.017 ± 0.098 | 0.015 ± 0.072 | 0.002 (-0.044, 0.048) | 0.928 | ns | 0.02 | no | ||
| 7 | 0.013 ± 0.073 | 0.013 ± 0.071 | 0.000 (-0.037, 0.037) | 0.982 | ns | -0.01 | no | ||
| 15 | 0.020 ± 0.052 | 0.010 ± 0.079 | 0.009 (-0.029, 0.047) | 0.631 | ns | 0.14 | no | ||
| 16 | 0.025 ± 0.037 | 0.011 ± 0.073 | 0.015 (-0.014, 0.043) | 0.300 | ns | 0.24 | small | ||
| 33 | 0.030 ± 0.046 | 0.031 ± 0.075 | -0.001 (-0.033, 0.031) | 0.957 | ns | -0.01 | no | ||
| FP | R | 12 | 0.008 ± 0.041 | 0.011 ± 0.042 | -0.003 (-0.026, 0.02) | 0.776 | ns | -0.08 | no |
| 19 | 0.014 ± 0.043 | 0.021 ± 0.049 | -0.007 (-0.031, 0.017) | 0.533 | ns | -0.16 | no | ||
| 20 | 0.008 ± 0.037 | 0.011 ± 0.044 | -0.003 (-0.027, 0.022) | 0.831 | ns | -0.06 | no | ||
| 21 | 0.009 ± 0.031 | 0.011 ± 0.047 | -0.003 (-0.024, 0.019) | 0.809 | ns | -0.07 | no | ||
| 28 | 0.025 ± 0.05 | 0.016 ± 0.058 | 0.008 (-0.02, 0.037) | 0.551 | ns | 0.16 | no | ||
| 29 | 0.019 ± 0.054 | 0.003 ± 0.062 | 0.016 (-0.013, 0.045) | 0.270 | ns | 0.27 | small | ||
| 36 | 0.046 ± 0.045 | 0.031 ± 0.055 | 0.015 (-0.012, 0.042) | 0.275 | ns | 0.29 | small | ||
| 37 | 0.030 ± 0.055 | 0.014 ± 0.074 | 0.016 (-0.024, 0.056) | 0.425 | ns | 0.24 | small | ||
| 38 | 0.017 ± 0.044 | 0.004 ± 0.066 | 0.013 (-0.019, 0.045) | 0.411 | ns | 0.23 | small | ||
| L | 14 | 0.020 ± 0.061 | -0.001 ± 0.09 | 0.021 (-0.029, 0.07) | 0.398 | ns | 0.27 | small | |
| 22 | 0.000 ± 0.036 | 0.004 ± 0.044 | -0.004 (-0.025, 0.018) | 0.725 | ns | -0.09 | no | ||
| 23 | 0.005 ± 0.053 | 0.015 ± 0.071 | -0.011 (-0.046, 0.025) | 0.547 | ns | -0.17 | no | ||
| 24 | 0.014 ± 0.035 | 0.017 ± 0.083 | -0.003 (-0.037, 0.031) | 0.849 | ns | -0.05 | no | ||
| 31 | 0.010 ± 0.056 | 0.004 ± 0.073 | 0.006 (-0.027, 0.039) | 0.700 | ns | 0.1 | no | ||
| 32 | 0.020 ± 0.056 | 0.018 ± 0.076 | 0.002 (-0.038, 0.043) | 0.901 | ns | 0.04 | no | ||
| 39 | 0.021 ± 0.044 | 0.015 ± 0.057 | 0.006 (-0.022, 0.033) | 0.670 | ns | 0.11 | no | ||
| 40 | 0.031 ± 0.062 | 0.017 ± 0.074 | 0.014 (-0.024, 0.052) | 0.459 | ns | 0.21 | small | ||
| 41 | 0.043 ± 0.056 | 0.039 ± 0.075 | 0.004 (-0.026, 0.034) | 0.782 | ns | 0.06 | no |
Table 7. Oxy-Hb changes during go/no-go task under music and no-music conditions in older group.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; SD: standard deviation; MD: mean difference; CI: confidence interval; p-value: p-value uncorrected; Sig: significance after Bonferroni correction; ns: not significant; R: right; L: left
| Area | Side | CH | Music (mM・cm) (mean ± SD) | No-music (mM・cm) (mean ± SD) | MD (95% CI) | p-value | Sig | Cohen‘s d | Effect size |
| DLPFC | R | 2 | 0.031 ± 0.039 | 0.009 ± 0.042 | 0.022 (0.004, 0.04) | 0.021 | ns | 0.53 | medium |
| 3 | 0.024 ± 0.037 | -0.008 ± 0.034 | 0.032 (0.016, 0.048) | 0.000 | sig | 0.90 | large | ||
| 4 | 0.011 ± 0.035 | 0.012 ± 0.033 | 0.000 (-0.02, 0.019) | 0.976 | ns | -0.01 | no | ||
| 10 | 0.046 ± 0.042 | 0.031 ± 0.046 | 0.015 (-0.005, 0.035) | 0.130 | ns | 0.34 | small | ||
| 11 | 0.043 ± 0.052 | 0.002 ± 0.052 | 0.041 (0.021, 0.062) | 0.000 | sig | 0.79 | medium | ||
| 27 | 0.056 ± 0.052 | 0.030 ± 0.056 | 0.026 (0.008, 0.044) | 0.006 | ns | 0.48 | small | ||
| L | 5 | 0.007 ± 0.027 | -0.001 ± 0.030 | 0.009 (-0.006, 0.024) | 0.231 | ns | 0.31 | small | |
| 6 | 0.026 ± 0.048 | -0.001 ± 0.049 | 0.026 (0.007, 0.046) | 0.011 | ns | 0.54 | medium | ||
| 7 | 0.024 ± 0.07 | 0.005 ± 0.039 | 0.02 (-0.007, 0.046) | 0.137 | ns | 0.34 | small | ||
| 15 | 0.045 ± 0.05 | 0.01 ± 0.043 | 0.036 (0.016, 0.056) | 0.001 | sig | 0.77 | medium | ||
| 16 | 0.044 ± 0.049 | 0.004 ± 0.038 | 0.04 (0.022, 0.058) | 0.000 | sig | 0.89 | large | ||
| 33 | 0.046 ± 0.04 | 0.029 ± 0.061 | 0.017 (-0.006, 0.04) | 0.141 | ns | 0.33 | small | ||
| FP | R | 12 | 0.024 ± 0.046 | -0.003 ± 0.034 | 0.027 (0.011, 0.042) | 0.002 | sig | 0.65 | medium |
| 19 | 0.049 ± 0.059 | 0.011 ± 0.061 | 0.037 (0.016, 0.059) | 0.001 | sig | 0.62 | medium | ||
| 20 | 0.037 ± 0.050 | 0.006 ± 0.046 | 0.03 (0.015, 0.046) | 0.000 | sig | 0.63 | medium | ||
| 21 | 0.023 ± 0.046 | -0.001 ± 0.036 | 0.023 (0.01, 0.036) | 0.001 | sig | 0.55 | medium | ||
| 28 | 0.057 ± 0.059 | 0.015 ± 0.070 | 0.042 (0.019, 0.065) | 0.001 | sig | 0.64 | medium | ||
| 29 | 0.039 ± 0.056 | 0.000 ± 0.050 | 0.04 (0.022, 0.057) | 0.000 | sig | 0.74 | medium | ||
| 36 | 0.052 ± 0.047 | 0.019 ± 0.052 | 0.034 (0.016, 0.052) | 0.001 | sig | 0.68 | medium | ||
| 37 | 0.062 ± 0.062 | 0.001 ± 0.054 | 0.061 (0.043, 0.079) | 0.000 | sig | 1.04 | large | ||
| 38 | 0.044 ± 0.049 | -0.016 ± 0.048 | 0.06 (0.041, 0.079) | 0.000 | sig | 1.23 | large | ||
| L | 14 | 0.039 ± 0.052 | -0.001 ± 0.046 | 0.04 (0.021, 0.058) | 0.000 | sig | 0.82 | large | |
| 22 | 0.027 ± 0.041 | -0.004 ± 0.035 | 0.031 (0.018, 0.044) | 0.000 | sig | 0.81 | large | ||
| 23 | 0.040 ± 0.044 | 0.008 ± 0.040 | 0.032 (0.012, 0.052) | 0.003 | ns | 0.75 | medium | ||
| 24 | 0.036 ± 0.038 | 0.006 ± 0.035 | 0.031 (0.017, 0.044) | 0.000 | sig | 0.83 | large | ||
| 31 | 0.051 ± 0.044 | 0.006 ± 0.051 | 0.045 (0.024, 0.066) | 0.000 | sig | 0.94 | large | ||
| 32 | 0.059 ± 0.046 | 0.017 ± 0.045 | 0.043 (0.022, 0.063) | 0.000 | sig | 0.93 | large | ||
| 39 | 0.047 ± 0.048 | -0.010 ± 0.044 | 0.057 (0.038, 0.077) | 0.000 | sig | 1.25 | large | ||
| 40 | 0.056 ± 0.049 | 0.010 ± 0.048 | 0.046 (0.026, 0.066) | 0.000 | sig | 0.94 | large | ||
| 41 | 0.049 ± 0.047 | 0.028 ± 0.050 | 0.021 (0.002, 0.04) | 0.028 | ns | 0.43 | small |
Table 8. Correlation between the changes in the accuracy of go/no-go test and oxy-Hb levels in 20 channels in which significant changes were observed between the music and no-music conditions in older adults.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; oxy-Hb: oxyhemoglobin; Sig: significance; ns: not significant
| Area | Side | CH | Pearson r | 95% CI | p-value | Sig |
| DLPFC | R | 3 | 0.105 | -0.259, 0.443 | 0.574 | ns |
| 11 | -0.144 | -0.469, 0.215 | 0.431 | ns | ||
| L | 15 | -0.293 | -0.583, 0.062 | 0.103 | ns | |
| 16 | -0.21 | -0.526, 0.155 | 0.256 | ns | ||
| FP | R | 12 | -0.473 | -0.708, -0.142 | 0.007 | sig |
| 19 | -0.16 | -0.482, 0.199 | 0.381 | ns | ||
| 20 | -0.16 | -0.482, 0.2 | 0.382 | ns | ||
| 21 | -0.429 | -0.676, -0.094 | 0.014 | sig | ||
| 28 | 0.043 | -0.31, 0.386 | 0.815 | ns | ||
| 29 | -0.258 | -0.556, 0.1 | 0.154 | ns | ||
| 36 | -0.114 | -0.45, 0.25 | 0.541 | ns | ||
| 37 | -0.217 | -0.526, 0.142 | 0.233 | ns | ||
| 38 | -0.31 | -0.612, 0.071 | 0.109 | ns | ||
| L | 14 | -0.461 | -0.698, -0.134 | 0.008 | sig | |
| 22 | -0.361 | -0.634, -0.007 | 0.046 | sig | ||
| 24 | -0.361 | -0.63, -0.014 | 0.043 | sig | ||
| 31 | -0.353 | -0.625, -0.005 | 0.047 | sig | ||
| 32 | -0.249 | -0.55, 0.109 | 0.169 | ns | ||
| 39 | -0.32 | -0.614, 0.053 | 0.091 | ns | ||
| 40 | -0.316 | -0.599, 0.037 | 0.078 | ns |
Figure 4. (A) Changes in oxy-Hb levels under the music and no-music conditions during the go/no-go task from the go task in the older group. (B) The location of the channels in which significant increases in oxy-Hb levels were observed under the music condition in older adults. The numbers in the circle indicate the channels.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; oxy-Hb: oxyhemoglobin
Error bars indicate 95% CI. *p < 0.05 adjusted using the Bonferroni method versus no-music condition.
Figure 5. (A) The significant negative correlation between changes in the task accuracy and changes in oxy-Hb levels under the music condition in the six channels in older adults. (B) The location of the channels in which a significant correlation between the decline in the task accuracy and increased oxy-Hb levels under the music condition were observed in older adults.
DLPFC: dorsolateral prefrontal cortex; FP: frontal pole; oxy-Hb: oxyhemoglobin
The numbers in the circle indicate the channels.
Discussion
This report demonstrated that background music during an inhibition task decreased inhibition control and significantly increased PFC activity in DLPFC and FP in the healthy older group but not in the young group. Furthermore, a significant negative correlation was observed between increased oxy-Hb levels in the FP and the decline in the accuracy response rate in the older group.
The present study showed that background music had a negative effect on cognitive task performance in the healthy older group but not in the younger group. Previous studies have assessed the negative effect of music on cognitive task performance and shown that relaxing music interferes with attentional control compared with stimulating music and silence [11]. Music has a distracting effect on novice surgeons performing new tasks in the operation theater [10]. Designers showed an improvement in their performance in alerting and orienting attention under no-music conditions compared with that under music conditions, indicating that an environment with music decreases the concentration of designers [37].
The negative effect of background music on cognitive tasks was observed in not younger but older groups in the present study. In contrast to the findings of the present study, some studies have shown no difference between younger and older adults in terms of the influences of music on attentional control [11,16]. Some studies using other cognitive tasks, such as memory performance, have shown that the executive function of older adults, compared with that of younger adults, declined due to music [15,38]. EI Haj et al. reported that older adults had a significant reduction in source memory after music exposure compared with young adults [38]. Reaves et al. showed that background music had impaired visual associative memory performance compared with silence in older adults but not in younger ones [15]. Given these findings, older adults might want to avoid listening to music when performing tasks that require intense attention.
Our findings revealed a significant increase in oxy-Hb levels in the PFC, which is associated with attention control, inhibition control, and dual-task performance [17-20], in the healthy older group but not in the younger group. Furthermore, a significant negative correlation was observed between increased oxy-Hb levels in the FP and the decline in accuracy response rate under the music condition in the older group. Previous studies have revealed that older adults exhibit greater brain activities than young adults during the selective inhibition task in the NIRS study [23,24]. In an fMRI study using the go/nogo task, older adults showed more brain activity during the task compared to young adults, and the increases in brain activation were related to good inhibitory performance [39]. An fMRI study using an antisaccade task revealed that older adults, not young ones, showed additional recruitment of the FP, which was correlated with faster antisaccade reaction times, and increased DLPFC activity, which was associated with better performance in inhibitory control [40]. Increased PFC activation in older adults has been explained as compensation for the reduced executive function [41,42]. It has also been explained using posterior to anterior sift theory in aging [43]. Meanwhile, increased PFC activation accompanied by aging reflects a less specific or efficient activity rather than compensation [44]. The present study revealed a negative correlation between PFC activity and response accuracy, thereby indicating that increased PFC activity was related to reduced response accuracy, which might be reflected as confused cognitive function due to background music in older adults.
Whether the music was specific for the impairment effect of inhibition control and PFC activation was unclear. The current study aimed to investigate the difference between with and without music. A previous study revealed that classical music does not affect cognitive functions, whereas white noise impairs these functions [45]. Another study showed that exposure to music, but not silence or street noise, interferes with cognitive functions, such as working memory [38]. Further research using control conditions with different types of music or sounds (e.g., white noise) must be conducted to determine the effect of different types of music or sounds on inhibition control and PFC activation in older adults.
The type of music and task difficulties might be associated with task performance decline while listening to background music. Music has several components, including tempo, rhythm, melody, lyrics, and mode, such as exciting, happy, and sad. Relaxing music has interfered with visuospatial attention [11]. The tempo of background music can influence concentration and performance [12,13,46]. Dovorany et al. reported that music with the major mode using a fast tempo and minor mode using a slow tempo modulated different aspects of attention after listening to music [46]. Music with a speed-up tempo can make drivers more excited and decrease their concentration, which leads to a less stable performance [12]. Listening to fast-tempo music is associated with increased mental load and reduced hazard perception ability during traffic among novice drivers; meanwhile, listening to slow-tempo music does not increase the mental load of novice drivers and can have some benefits to hazard perception [13]. The current study could not validate the influences of melody tempo because it was set at a stable value of 120 bpm. Further studies using various tempos of melody should be performed.
A previous study showed that preferred background music can enhance task-focused attentional status on a low-demanding sustained-attention task in students [8]. Qiu et al. have revealed that music promotes brain activity, particularly in the PFC, and the activation induced by personally preferred music is more robust than that of neutral music in an EEG-fNIRS study [47]. In the present study, we did not investigate the degree of preference for the music. Further research should be performed to validate the effects of music on executive function and how PFC activity can be influenced by music preference in older adults.
Herein, the inhibitory control and PFC activation differed between healthy younger and older adults based on an analysis using a relatively simple, noninvasive method. Some types of go/no-go tasks were used in the previous studies [5-7,48]. The go/no-go task using color was simpler than that using images such as animal figures. NIRS is a noninvasive technique to monitor cerebral activity and is associated with less physical restriction than fMRI. Reportedly, a decline in attention and inhibitory control can predict future accidents in older adults [3,4]. Higher levels of PFC activation during the dual task can predict falls in older adults [49]. Herein, performing tasks while listening to background music might work as a dual task and interfere with task performance. Therefore, a decline in inhibitory task performance and an increase in PFC activity while listening to background music may predict future accidents in older adults. Further studies must evaluate the association between a decline in inhibitory control and an increase in PFC activity, as observed in older adults herein, as well as future accidents.
This study had some limitations in terms of result interpretation. That is, the degree of concentration for listening to background music could not be quantified, although the participants were instructed to listen to it carefully under the music condition. As mentioned above, whether other types of music or tempo influence the inhibition of control performance and PFC activation remains unclear. Further research should be performed to validate these issues.
Conclusions
Listening to background music reduced the accuracy of inhibition task responses in older but not younger people. It also increased the PFC activation associated with attention and inhibitory function. Thus, background music is an interference stimulus, and it can decrease attention and significantly increase PFC activity in older adults. Older people might want to avoid listening to music when performing tasks requiring strong attention. Further large-scale cohort studies must be performed to validate our results.
Acknowledgments
The authors thank all participants in this study. The authors also thank Ms. Ami Ikeba, speech-language-hearing therapist (ST), and ST team members of the Department of Rehabilitation in Chubu Medical Center for performing neuropsychologic examinations, and Mr. Shogo Sawamura for useful discussions. The authors also thank Enago (www.enago.jp) for the English language review. Yuka Okumura and Jun Matsumoto-Miyazaki contributed equally to this work and should be considered co-first authors. The datasets generated for this article are not readily available for ethical and privacy reasons. However, data are available on reasonable request. The data are stored as de-identified participant data which are available on request to jmiyamatsu@gmail.com.
Funding Statement
A part of this study was funded by ZENKYOREN (National Mutual Insurance Federation of Agricultural Cooperatives) in Japan (0014-2020). The ZENKYOREN had no role in the design, conduct of the study, management, interpretation of the data, and preparation, review, and approval of the manuscript.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Ethics Committee of Kizawa Memorial Hospital issued approval 2020-003.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: A part of this study was funded by ZENKYOREN (National Mutual Insurance Federation of Agricultural Cooperatives) in Japan (0014-2020). The ZENKYOREN had no role in the design, conduct of the study, management, interpretation of the data, and preparation, review, and approval of the manuscript.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Jun Matsumoto-Miyazaki, Yuka Okumura, Yoshitaka Asano, Masaru Makibayashi, Jun Shinoda, Hirohito Yano
Acquisition, analysis, or interpretation of data: Jun Matsumoto-Miyazaki, Yuka Okumura, Yuka Ikegame
Drafting of the manuscript: Jun Matsumoto-Miyazaki, Yuka Okumura
Critical review of the manuscript for important intellectual content: Jun Matsumoto-Miyazaki, Yuka Okumura, Yuka Ikegame, Yoshitaka Asano, Masaru Makibayashi, Jun Shinoda, Hirohito Yano
Supervision: Jun Shinoda, Hirohito Yano
References
- 1.Executive functions. Diamond A. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Executive function abilities in cognitively healthy young and older adults - a cross-sectional study. Idowu MI, Szameitat AJ. Front Aging Neurosci. 2023;15:976915. doi: 10.3389/fnagi.2023.976915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. J Gerontol A Biol Sci Med Sci. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. Mirelman A, Herman T, Brozgol M, et al. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effects of relaxing music on mental fatigue induced by a continuous performance task: behavioral and ERPs evidence. Guo W, Ren J, Wang B, Zhu Q. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0136446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Monden Y, Dan H, Nagashima M, et al. Clin Neurophysiol. 2012;123:1147–1157. doi: 10.1016/j.clinph.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 7.The effect of background music on inhibitory functions: an ERP study. Burkhard A, Elmer S, Kara D, Brauchli C, Jäncke L. Front Hum Neurosci. 2018;12:293. doi: 10.3389/fnhum.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The effect of preferred background music on task-focus in sustained attention. Kiss L, Linnell KJ. Psychol Res. 2021;85:2313–2325. doi: 10.1007/s00426-020-01400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Background music: effects on attention performance. Shih YN, Huang RH, Chiang HY. Work. 2012;42:573–578. doi: 10.3233/WOR-2012-1410. [DOI] [PubMed] [Google Scholar]
- 10.Randomized controlled trial investigating the effect of music on the virtual reality laparoscopic learning performance of novice surgeons. Miskovic D, Rosenthal R, Zingg U, Oertli D, Metzger U, Jancke L. Surg Endosc. 2008;22:2416–2420. doi: 10.1007/s00464-008-0040-8. [DOI] [PubMed] [Google Scholar]
- 11.Effect of background music on attentional control in older and young adults. Cloutier A, Fernandez NB, Houde-Archambault C, Gosselin N. Front Psychol. 2020;11:557225. doi: 10.3389/fpsyg.2020.557225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Is music a mediator impacting car following when driver's personalities are considered. Niu J, Ma C, Liu J, Li L, Hu T, Ran L. Accid Anal Prev. 2020;147:105774. doi: 10.1016/j.aap.2020.105774. [DOI] [PubMed] [Google Scholar]
- 13.The influence of music tempo on mental load and hazard perception of novice drivers. Miao L, Gu Y, He L, Wang H, Schwebel DC, Shen Y. Accid Anal Prev. 2021;157:106168. doi: 10.1016/j.aap.2021.106168. [DOI] [PubMed] [Google Scholar]
- 14.The impact of background music on adult listeners: a meta-analysis. Kämpfe J, Sedlmeier P, Renkewitz F. Psychol Music. 2011;39:424–448. [Google Scholar]
- 15.Turn off the music! Music impairs visual associative memory performance in older adults. Reaves S, Graham B, Grahn J, Rabannifard P, Duarte A. Gerontologist. 2016;56:569–577. doi: 10.1093/geront/gnu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brain networks mediating the influence of background music on selective attention. Fernandez NB, Trost WJ, Vuilleumier P. Soc Cogn Affect Neurosci. 2019;14:1441–1452. doi: 10.1093/scan/nsaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The role of the anterior prefrontal cortex in human cognition. Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 18.Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Ramnani N, Owen AM. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 19.Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: a systematic review of an emerging area of research. Agbangla NF, Audiffren M, Albinet CT. Ageing Res Rev. 2017;38:52–66. doi: 10.1016/j.arr.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: a review. Udina C, Avtzi S, Durduran T, et al. Front Aging Neurosci. 2019;11:367. doi: 10.3389/fnagi.2019.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapid event-related near-infrared spectroscopy detects age-related qualitative changes in the neural correlates of response inhibition. Heilbronner U, Münte TF. Neuroimage. 2013;65:408–415. doi: 10.1016/j.neuroimage.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 22.Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Spreng RN, Wojtowicz M, Grady CL. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Do older adults mistake the accelerator for the brake pedal?: older adults employ greater prefrontal cortical activity during a bipedal/bimanual response-position selection task. Kawai N, Nakata R. https://doi.org/10.1016/j.bbr.2022.113976. Behav Brain Res. 2022;432:113976. doi: 10.1016/j.bbr.2022.113976. [DOI] [PubMed] [Google Scholar]
- 24.Older adults exhibit greater brain activity than young adults in a selective inhibition task by bipedal and bimanual responses: an fNIRS study. Kawai N, Nakata R, Kubo-Kawai N. Neuroreport. 2020;31:1048–1053. doi: 10.1097/WNR.0000000000001516. [DOI] [PubMed] [Google Scholar]
- 25.The revised Hasegawa’s dementia Scale (HDS-R)-evaluation of its usefulness as a screening test for dementia. Imai Y, Hasegawa K. J Hong Kong Coll Psychiatr. 1994;4:20–24. [Google Scholar]
- 26.“Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Folstein MF, Folstein SE, McHugh PR. J Psychiatr Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Petersen RC, Aisen PS, Beckett LA, et al. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyougei Music Research Group. Uta Ha Tomodachi (Music Is Friend) Tokyo: Kyoikugeijutsu sha; 2016. [Google Scholar]
- 29.System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Cope M, Delpy DT. Med Biol Eng Comput. 1988;26:289–294. doi: 10.1007/BF02447083. [DOI] [PubMed] [Google Scholar]
- 30.A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Strangman G, Culver JP, Thompson JH, et al. https://doi.org/10.1006/nimg.2002.1227. NeuroImage. 2002;17:719–731. [PubMed] [Google Scholar]
- 31.fMRI validation of fNIRS measurements during a naturalistic task. Noah JA, Ono Y, Nomoto Y, et al. J Vis Exp. 2015:0. doi: 10.3791/52116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Differential prefrontal and frontotemporal oxygenation patterns during phonemic and semantic verbal fluency. Tupak SV, Badewien M, Dresler T, et al. https://doi.org/10.1016/j.neuropsychologia.2012.03.009. Neuropsychologia. 2012;50:1565–1569. doi: 10.1016/j.neuropsychologia.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Neural correlates of a standardized version of the trail making test in young and elderly adults: a functional near-infrared spectroscopy study. Müller LD, Guhn A, Zeller JB, et al. https://doi.org/10.1016/j.neuropsychologia.2014.01.019. Neuropsychologia. 2014;56:271–279. doi: 10.1016/j.neuropsychologia.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Compromised brain activity with age during a game-like dynamic balance task: single- vs. dual-task performance. de Rond V, Orcioli-Silva D, Dijkstra BW, Orban de Xivry JJ, Pantall A, Nieuwboer A. Front Aging Neurosci. 2021;13:657308. doi: 10.3389/fnagi.2021.657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans JD. Belmont: Thomson Brooks/Cole Publishing Co.; 1996. Straightforward Statistics for the Behavioral Sciences. [Google Scholar]
- 36.Two-dimensional ultrasound shear wave elastography for identifying and staging liver fibrosis in pediatric patients with known or suspected liver disease: a clinical effectiveness study. Alhashmi GH, Gupta A, Trout AT, Dillman JR. Pediatr Radiol. 2020;50:1255–1262. doi: 10.1007/s00247-020-04720-2. [DOI] [PubMed] [Google Scholar]
- 37.The effect of musical environments on designers’ attention: persistent music listening interferes with attention. Yu S, Chen X. Behav Sci (Basel) 2024;14 doi: 10.3390/bs14030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Music causes deterioration of source memory: evidence from normal ageing. El Haj M, Omigie D, Clément S. Q J Exp Psychol (Hove) 2014;67:2381–2391. doi: 10.1080/17470218.2014.929719. [DOI] [PubMed] [Google Scholar]
- 39.Frontal recruitment during response inhibition in older adults replicated with fMRI. Langenecker SA, Nielson KA. Neuroimage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- 40.Age related prefrontal compensatory mechanisms for inhibitory control in the antisaccade task. Fernandez-Ruiz J, Peltsch A, Alahyane N, Brien DC, Coe BC, Garcia A, Munoz DP. Neuroimage. 2018;165:92–101. doi: 10.1016/j.neuroimage.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Neurocognitive aging and the compensation hypothesis. Reuter-Lorenz PA, Cappell KA. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- 42.The adaptive brain: aging and neurocognitive scaffolding. Park DC, Reuter-Lorenz P. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Que PASA? The posterior-anterior shift in aging. Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. Morcom AM, Henson RN. J Neurosci. 2018;38:7303–7313. doi: 10.1523/JNEUROSCI.1701-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dissociable effects of music and white noise on conflict-induced behavioral adjustments. Pascoe AJ, Haque ZZ, Samandra R, Fehring DJ, Mansouri FA. Front Neurosci. 2022;16:858576. doi: 10.3389/fnins.2022.858576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Happy and sad music acutely modulate different types of attention in older adults. Dovorany N, Brannick S, Johnson N, Ratiu I, LaCroix AN. Front Psychol. 2023;14:1029773. doi: 10.3389/fpsyg.2023.1029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Multi-modal integration of EEG-fNIRS for characterization of brain activity evoked by preferred music. Qiu L, Zhong Y, Xie Q, et al. Front Neurorobot. 2022;16:823435. doi: 10.3389/fnbot.2022.823435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The influence of music tempo on inhibitory control: an ERP study. Xiao R, Liu C, Chen J, Chen J. Front Behav Neurosci. 2020;14:48. doi: 10.3389/fnbeh.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brain activation in high-functioning older adults and falls: prospective cohort study. Verghese J, Wang C, Ayers E, Izzetoglu M, Holtzer R. Neurology. 2017;88:191–197. doi: 10.1212/WNL.0000000000003421. [DOI] [PMC free article] [PubMed] [Google Scholar]