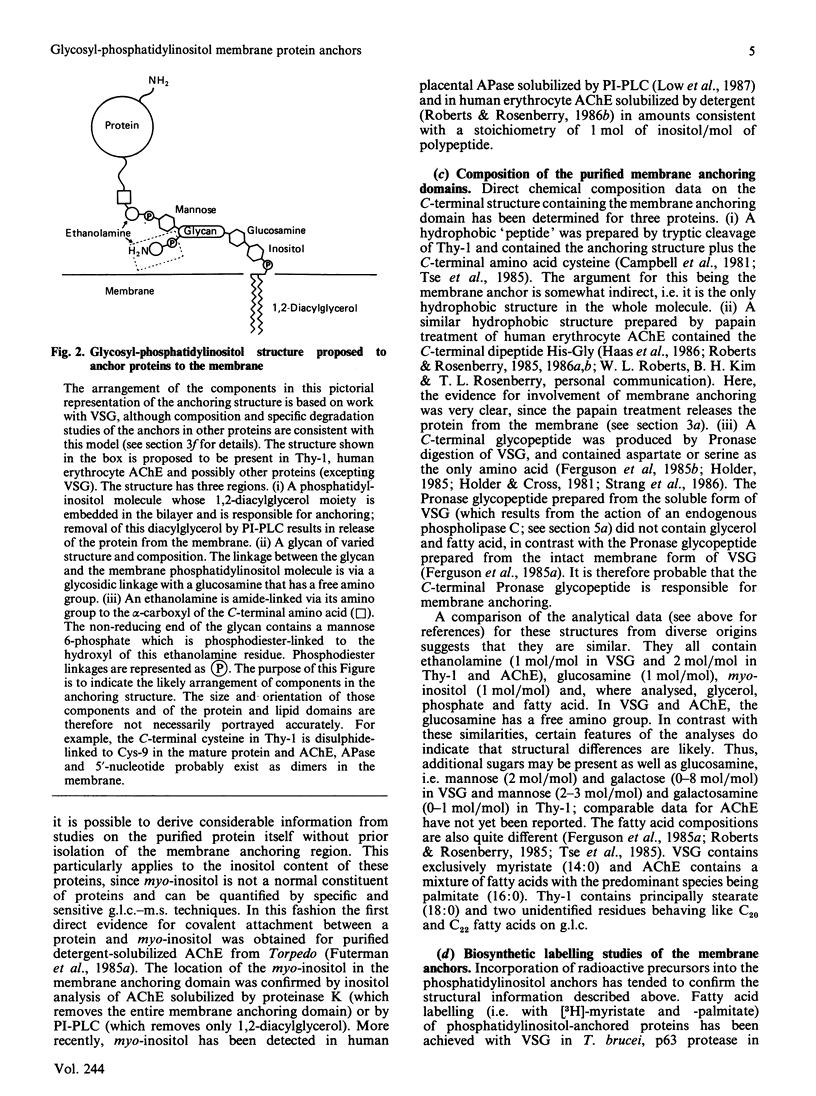
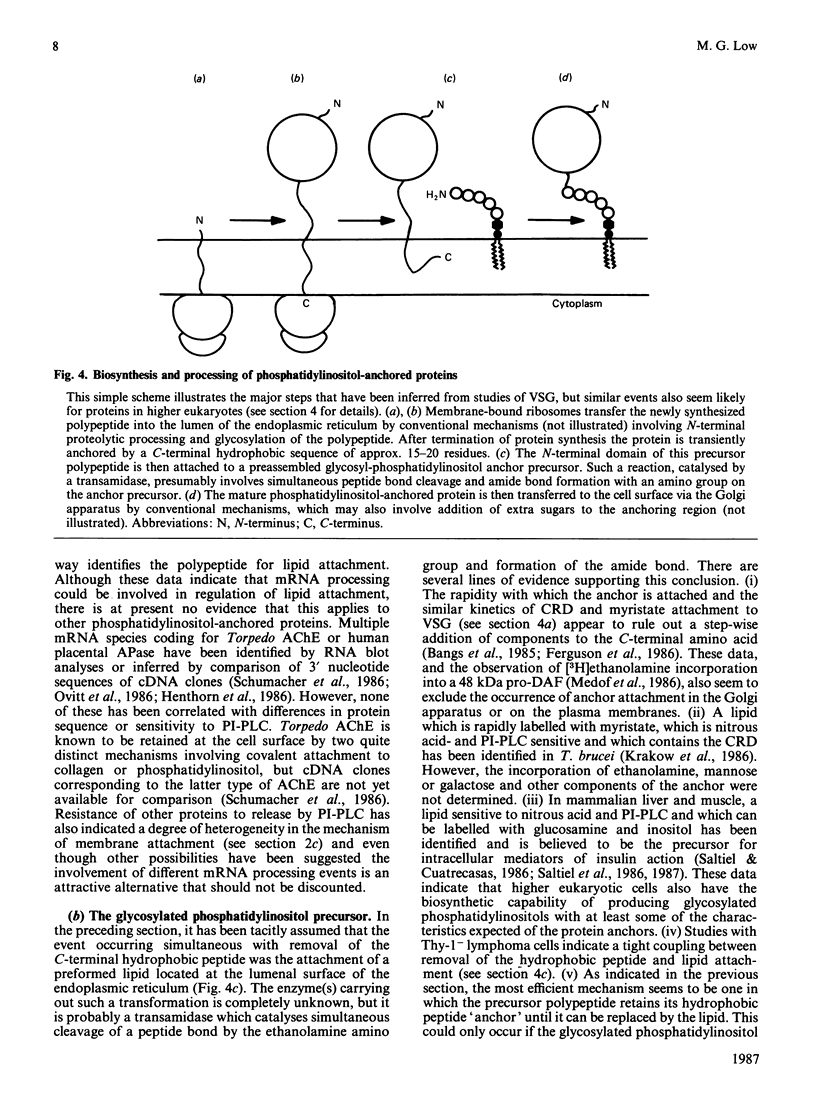
Full text
PDF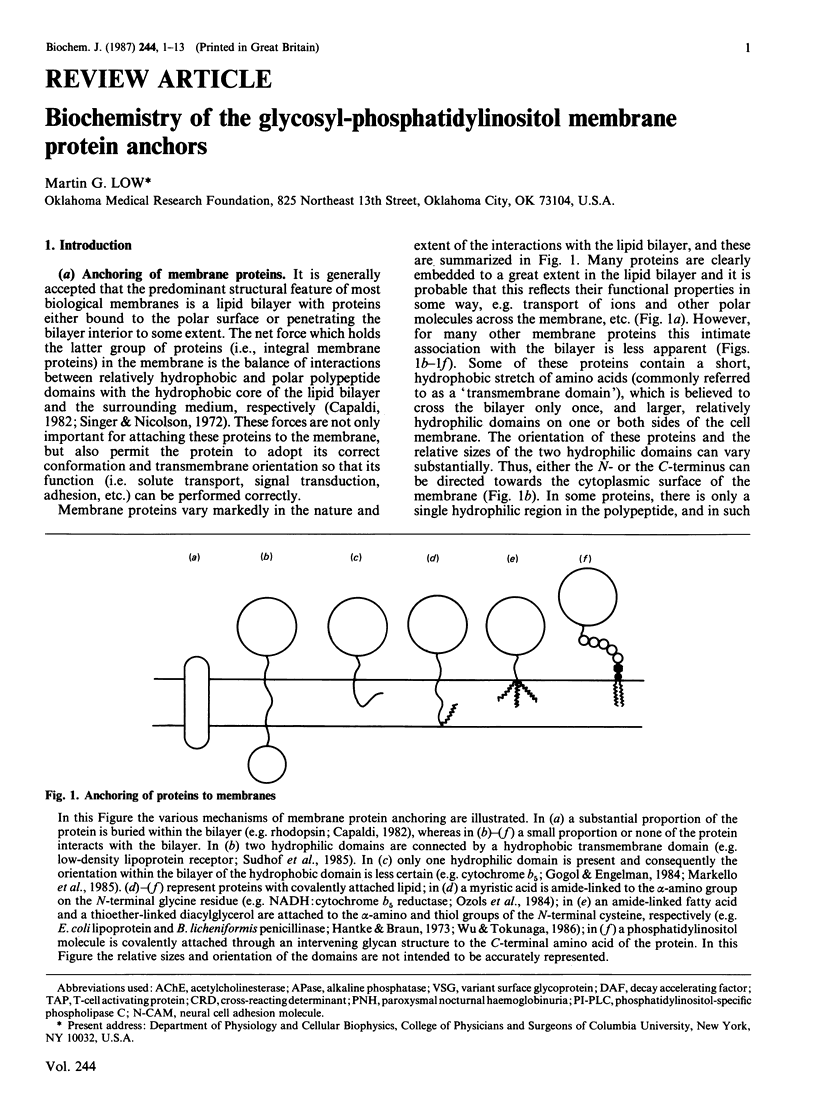










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Hasan N. S., Sutcliffe R. G. Molecular heterogeneity of human placental alkaline phosphatase associated with microvillous membranes. Prog Clin Biol Res. 1984;166:117–126. [PubMed] [Google Scholar]
- Bangs J. D., Andrews N. W., Hart G. W., Englund P. T. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J Cell Biol. 1986 Jul;103(1):255–263. doi: 10.1083/jcb.103.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. D., Pope B., Luzio J. P. The membrane topography of ecto-5'-nucleotidase in rat hepatocytes. Biochem J. 1986 Jun 1;236(2):495–502. doi: 10.1042/bj2360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Suchard S. J., Kalomiris E. L. Lymphoma Thy-1 glycoprotein is linked to the cytoskeleton via a 4.1-like protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2529–2540. doi: 10.1083/jcb.103.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Voorheis H. P. Release of the surface coat from the plasma membrane of intact bloodstream forms of Trypanosoma brucei requires Ca2+. FEBS Lett. 1982 Mar 8;139(1):17–21. doi: 10.1016/0014-5793(82)80477-8. [DOI] [PubMed] [Google Scholar]
- Bülow R., Overath P. Purification and characterization of the membrane-form variant surface glycoprotein hydrolase of Trypanosoma brucei. J Biol Chem. 1986 Sep 5;261(25):11918–11923. [PubMed] [Google Scholar]
- Bülow R., Overath P. Synthesis of a hydrolase for the membrane-form variant surface glycoprotein is repressed during transformation of Trypanosoma brucei. FEBS Lett. 1985 Jul 22;187(1):105–110. doi: 10.1016/0014-5793(85)81223-0. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capceville Y., Baltz T., Deregnaucourt C., Keller A. M. Immunological evidence of a common structure between Paramecium surface antigens and Trypanosoma variant surface glycoproteins. Exp Cell Res. 1986 Nov;167(1):75–86. doi: 10.1016/0014-4827(86)90205-3. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Chow F. L., Telen M. J., Rosse W. F. The acetylcholinesterase defect in paroxysmal nocturnal hemoglobinuria: evidence that the enzyme is absent from the cell membrane. Blood. 1985 Oct;66(4):940–945. [PubMed] [Google Scholar]
- Colbeau A., Maroux S. Integration of alkaline phosphatase in the intestinal brush border membrane. Biochim Biophys Acta. 1978 Jul 20;511(1):39–51. doi: 10.1016/0005-2736(78)90063-9. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Jacob H. S. Complement-mediated granulocyte dysfunction in paroxysmal nocturnal hemoglobinuria. Blood. 1976 Jun;47(6):931–939. [PubMed] [Google Scholar]
- Creek K. E., Rimoldi D., Clifford A. J., Silverman-Jones C. S., De Luca L. M. Mannosylation of endogenous and exogenous phosphatidic acid by liver microsomal membranes. Formation of phosphatidylmannose. J Biol Chem. 1986 Mar 15;261(8):3490–3500. [PubMed] [Google Scholar]
- Cross G. A. Crossreacting determinants in the C-terminal region of trypanosome variant surface antigens. Nature. 1979 Jan 25;277(5694):310–312. doi: 10.1038/277310a0. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Structure of the variant glycoproteins and surface coat of Trypanosoma brucei. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):3–12. doi: 10.1098/rstb.1984.0104. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Gurnett A. M., Low M. G., Turner M. J., Nussenzweig V. Decay-accelerating factor (DAF) shares a common carbohydrate determinant with the variant surface glycoprotein (VSG) of the African Trypanosoma brucei. J Immunol. 1987 Jan 15;138(2):520–523. [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Weiss E. H., Paulson M., Flavell R. A. Duplicated gene pairs and alleles of class I genes in the Qa2 region of the murine major histocompatibility complex: a comparison. EMBO J. 1985 Dec 1;4(12):3203–3207. doi: 10.1002/j.1460-2075.1985.tb04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragsten P., Henkart P., Blumenthal R., Weinstein J., Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta-Choudhury T. A., Rosenberry T. L. Human erythrocyte acetylcholinesterase is an amphipathic protein whose short membrane-binding domain is removed by papain digestion. J Biol Chem. 1984 May 10;259(9):5653–5660. [PubMed] [Google Scholar]
- Duvillier G., Nouvelot A., Richet C., Baltz T., Degand P. Presence of glycerol and fatty acids in the C-terminal end of a variant surface glycoprotein from Trypanosoma equiperdum. Biochem Biophys Res Commun. 1983 Jul 18;114(1):119–125. doi: 10.1016/0006-291x(83)91602-9. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986 Jul 15;261(20):9098–9101. [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Cross G. A. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3011–3015. [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Fliesler S. J., Anderson R. E. In vivo incorporation of [2-3H]-myo-inositol into frog opsin. Biochem Biophys Res Commun. 1986 Apr 29;136(2):815–821. doi: 10.1016/0006-291x(86)90513-9. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Duszenko M., Ferguson M. A., Low M. G., Cross G. A. Purification and characterization of a novel glycan-phosphatidylinositol-specific phospholipase C from Trypanosoma brucei. J Biol Chem. 1986 Nov 25;261(33):15767–15771. [PubMed] [Google Scholar]
- Futerman A. H., Fiorini R. M., Roth E., Low M. G., Silman I. Physicochemical behaviour and structural characteristics of membrane-bound acetylcholinesterase from Torpedo electric organ. Effect of phosphatidylinositol-specific phospholipase C. Biochem J. 1985 Mar 1;226(2):369–377. doi: 10.1042/bj2260369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Ackermann K. E., Sherman W. R., Silman I. Identification of covalently bound inositol in the hydrophobic membrane-anchoring domain of Torpedo acetylcholinesterase. Biochem Biophys Res Commun. 1985 May 31;129(1):312–317. doi: 10.1016/0006-291x(85)91439-1. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Michaelson D. M., Silman I. Solubilization of membrane-bound acetylcholinesterase by a phosphatidylinositol-specific phospholipase C. J Neurochem. 1985 Nov;45(5):1487–1494. doi: 10.1111/j.1471-4159.1985.tb07217.x. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Silman I. A hydrophobic dimer of acetylcholinesterase from Torpedo californica electric organ is solubilized by phosphatidylinositol-specific phospholipase C. Neurosci Lett. 1983 Sep 19;40(1):85–89. doi: 10.1016/0304-3940(83)90097-6. [DOI] [PubMed] [Google Scholar]
- Gaston S. M., Marchase R. B., Jakoi E. R. Brain ligatin: a membrane lectin that binds acetylcholinesterase. J Cell Biochem. 1982;18(4):447–459. doi: 10.1002/jcb.1982.240180406. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Gogol E. P., Engelman D. M. Neutron scattering shows that cytochrome b5 penetrates deeply into the lipid bilayer. Biophys J. 1984 Oct;46(4):491–495. doi: 10.1016/S0006-3495(84)84046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Haldar K., Ferguson M. A., Cross G. A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J Biol Chem. 1985 Apr 25;260(8):4969–4974. [PubMed] [Google Scholar]
- Haldar K., Henderson C. L., Cross G. A. Identification of the parasite transferrin receptor of Plasmodium falciparum-infected erythrocytes and its acylation via 1,2-diacyl-sn-glycerol. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8565–8569. doi: 10.1073/pnas.83.22.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- He H. T., Barbet J., Chaix J. C., Goridis C. Phosphatidylinositol is involved in the membrane attachment of NCAM-120, the smallest component of the neural cell adhesion molecule. EMBO J. 1986 Oct;5(10):2489–2494. doi: 10.1002/j.1460-2075.1986.tb04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Edelman G. M., Cunningham B. A. cDNA clones of the neural cell adhesion molecule (N-CAM) lacking a membrane-spanning region consistent with evidence for membrane attachment via a phosphatidylinositol intermediate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9822–9826. doi: 10.1073/pnas.83.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Knoll B. J., Raducha M., Rothblum K. N., Slaughter C., Weiss M., Lafferty M. A., Fischer T., Harris H. Products of two common alleles at the locus for human placental alkaline phosphatase differ by seven amino acids. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5597–5601. doi: 10.1073/pnas.83.15.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereld D., Krakow J. L., Bangs J. D., Hart G. W., Englund P. T. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986 Oct 15;261(29):13813–13819. [PubMed] [Google Scholar]
- Holder A. A. Carbohydrate is linked through ethanolamine to the C-terminal amino acid of Trypanosoma brucei variant surface glycoprotein. Biochem J. 1983 Jan 1;209(1):261–262. doi: 10.1042/bj2090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Cross G. A. Glycopeptides from variant surface glycoproteins of Trypanosoma Brucei. C-terminal location of antigenically cross-reacting carbohydrate moieties. Mol Biochem Parasitol. 1981 Feb;2(3-4):135–150. doi: 10.1016/0166-6851(81)90095-5. [DOI] [PubMed] [Google Scholar]
- Holder A. A. Glycosylation of the variant surface antigens of Trypanosoma brucei. Curr Top Microbiol Immunol. 1985;117:57–74. doi: 10.1007/978-3-642-70538-0_3. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Hyman R. Genetic basis for Ly-6- defect: complementation between Ly-6- and Thy-1- mutant cell lines. Immunogenetics. 1983;17(3):261–270. doi: 10.1007/BF00364410. [DOI] [PubMed] [Google Scholar]
- Hyman R. Cell-surface-antigen mutants of haematopoietic cells. Tools to study differentiation, biosynthesis and function. Biochem J. 1985 Jan 1;225(1):27–40. doi: 10.1042/bj2250027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Jemmerson R., Shah N., Takeya M., Fishman W. H. Functional organization of the placental alkaline phosphatase polypeptide chain. Prog Clin Biol Res. 1984;166:105–115. [PubMed] [Google Scholar]
- Kam W., Clauser E., Kim Y. S., Kan Y. W., Rutter W. J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Thiele H. G., Low M. G. Release of the rat T cell alloantigen RT-6.2 from cell membranes by phosphatidylinositol-specific phospholipase C. J Exp Med. 1986 Oct 1;164(4):1338–1343. doi: 10.1084/jem.164.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami T., Miki A., Ikehara Y. Electrophoretic characterization of hepatic alkaline phosphatase released by phosphatidylinositol-specific phospholipase C. A comparison with liver membrane and serum-soluble forms. Biochem J. 1985 Apr 1;227(1):183–189. doi: 10.1042/bj2270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. L., Hereld D., Bangs J. D., Hart G. W., Englund P. T. Identification of a glycolipid precursor of the Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1986 Sep 15;261(26):12147–12153. [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- LeClair K. P., Palfree R. G., Flood P. M., Hammerling U., Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986 Dec 1;5(12):3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. R., Murray E., Manolagas S., Olefsky J. M. Demonstration of insulin receptors and modulation of alkaline phosphatase activity by insulin in rat osteoblastic cells. Endocrinology. 1986 Oct;119(4):1786–1792. doi: 10.1210/endo-119-4-1786. [DOI] [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Cox A. C. Characterization of multiple forms of phosphoinositide-specific phospholipase C purified from human platelets. Biochem J. 1986 Jul 1;237(1):139–145. doi: 10.1042/bj2370139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Non-lytic release of acetylcholinesterase from erythrocytes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1977 Oct 1;82(1):143–146. doi: 10.1016/0014-5793(77)80905-8. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Release of alkaline phosphatase from membranes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1977 Oct 1;167(1):281–284. doi: 10.1042/bj1670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Specific release of plasma membrane enzymes by a phosphatidylinositol-specific phospholipase C. Biochim Biophys Acta. 1978 Apr 20;508(3):565–570. doi: 10.1016/0005-2736(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Low M. G., Futerman A. H., Ackermann K. E., Sherman W. R., Silman I. Removal of covalently bound inositol from Torpedo acetylcholinesterase and mammalian alkaline phosphatases by deamination with nitrous acid. Evidence for a common membrane-anchoring structure. Biochem J. 1987 Jan 15;241(2):615–619. doi: 10.1042/bj2410615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Weglicki W. B. Resolution of myocardial phospholipase C into several forms with distinct properties. Biochem J. 1983 Nov 1;215(2):325–334. doi: 10.1042/bj2150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Zilversmit D. B. Role of phosphatidylinositol in attachment of alkaline phosphatase to membranes. Biochemistry. 1980 Aug 19;19(17):3913–3918. doi: 10.1021/bi00558a004. [DOI] [PubMed] [Google Scholar]
- Maeda K., Ohta Y., Nakabayashi T., Taguchi R., Ikezawa H. Acid ATPase from chicken liver lysosomes. III. A metal ion-activated ATPase combines with membranous phosphatidylinositol. Biochem Int. 1986 Jun;12(6):855–863. [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Schlesinger M. J. Fatty acid acylation of eucaryotic cell membrane proteins. Biochim Biophys Acta. 1982 Nov 30;694(3):279–289. doi: 10.1016/0304-4157(82)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar R., Balasubramanian A. S. Essential and non-essential phosphatidylinositol residues in acetylcholinesterase and arylacylamidase of sheep basal ganglia. FEBS Lett. 1982 Sep 20;146(2):335–338. doi: 10.1016/0014-5793(82)80947-2. [DOI] [PubMed] [Google Scholar]
- Majumdar R., Balasubramanian A. S. The solubilization of platelet membrane-bound acetylcholinesterase and aryl acylamidase by exogenous or endogenous phosphatidylinositol specific phospholipase C. Biochem Pharmacol. 1985 Dec 1;34(23):4109–4115. doi: 10.1016/0006-2952(85)90202-3. [DOI] [PubMed] [Google Scholar]
- Malik A. S., Low M. G. Conversion of human placental alkaline phosphatase from a high Mr form to a low Mr form during butanol extraction. An investigation of the role of endogenous phosphoinositide-specific phospholipases. Biochem J. 1986 Dec 1;240(2):519–527. doi: 10.1042/bj2400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markello T., Zlotnick A., Everett J., Tennyson J., Holloway P. W. Determination of the topography of cytochrome b5 in lipid vesicles by fluorescence quenching. Biochemistry. 1985 Jun 4;24(12):2895–2901. doi: 10.1021/bi00333a012. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Miki A., Kominami T., Ikehara Y. pH-dependent conversion of liver-membranous alkaline phosphatase to a serum-soluble form by n-butanol extraction. Biochem Biophys Res Commun. 1985 Jan 16;126(1):89–95. doi: 10.1016/0006-291x(85)90575-3. [DOI] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- Mizobe F., Iwamoto M., Livett B. G. Parallel but separate release of catecholamines and acetylcholinesterase from stimulated adrenal chromaffin cells in culture. J Neurochem. 1984 May;42(5):1433–1438. doi: 10.1111/j.1471-4159.1984.tb02805.x. [DOI] [PubMed] [Google Scholar]
- Moriuchi T., Silver J. Rat Thy-1 antigen has a hydrophobic segment at the carboxyl terminus. FEBS Lett. 1984 Dec 3;178(1):105–108. doi: 10.1016/0014-5793(84)81250-8. [DOI] [PubMed] [Google Scholar]
- Nakabayashi T., Ikezawa H. Alkaline phosphodiesterase I release from eucaryotic plasma membranes by phosphatidylinositol-specific phospholipase C. I. The release from rat organs. J Biochem. 1986 Mar;99(3):703–712. doi: 10.1093/oxfordjournals.jbchem.a135529. [DOI] [PubMed] [Google Scholar]
- Nakabayashi T., Ikezawa H. Release of alkaline phosphodiesterase I from rat kidney plasma membrane produced by the phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Cell Struct Funct. 1984 Sep;9(3):247–263. doi: 10.1247/csf.9.247. [DOI] [PubMed] [Google Scholar]
- Overath P., Czichos J., Stock U., Nonnengaesser C. Repression of glycoprotein synthesis and release of surface coat during transformation of Trypanosoma brucei. EMBO J. 1983;2(10):1721–1728. doi: 10.1002/j.1460-2075.1983.tb01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovitt C. E., Strauss A. W., Alpers D. H., Chou J. Y., Boime I. Expression of different-sized placental alkaline phosphatase mRNAs in placenta and choriocarcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3781–3785. doi: 10.1073/pnas.83.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Carr S. A., Strittmatter P. Identification of the NH2-terminal blocking group of NADH-cytochrome b5 reductase as myristic acid and the complete amino acid sequence of the membrane-binding domain. J Biol Chem. 1984 Nov 10;259(21):13349–13354. [PubMed] [Google Scholar]
- Palfree R. G., Hämmerling U. Biochemical characterization of the murine activated lymphocyte alloantigen Ly-6E.1 controlled by the Ly-6 locus. J Immunol. 1986 Jan;136(2):594–600. [PubMed] [Google Scholar]
- Panagia V., Michiel D. F., Dhalla K. S., Nijjar M. S., Dhalla N. S. Role of phosphatidylinositol in basal adenylate cyclase activity of rat heart sarcolemma. Biochim Biophys Acta. 1981 Sep 4;676(3):395–400. doi: 10.1016/0304-4165(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Parker C. J., Soldato C. M., Rosse W. F. Abnormality of glycophorin-alpha on paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1984 Apr;73(4):1130–1143. doi: 10.1172/JCI111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Rifkin M. R., Fairlamb A. H. Transport of ethanolamine and its incorporation into the variant surface glycoprotein of bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1985 Jun;15(3):245–256. doi: 10.1016/0166-6851(85)90088-x. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Rosenberry T. L. Identification of covalently attached fatty acids in the hydrophobic membrane-binding domain of human erythrocyte acetylcholinesterase. Biochem Biophys Res Commun. 1985 Dec 17;133(2):621–627. doi: 10.1016/0006-291x(85)90950-7. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Rosenberry T. L. Selective radiolabeling and isolation of the hydrophobic membrane-binding domain of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3091–3098. doi: 10.1021/bi00359a004. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W., LOGAN G. F., Jr Mechanism of action of the toxin of Bacillus anthracis. I. Effect in vivo on some blood serum components. J Bacteriol. 1960 Jul;80:77–85. doi: 10.1128/jb.80.1.77-85.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEIN M. W., LOGAN G. F., Jr Partial purification and properties of two phospholipases of Bacillus cereus. J Bacteriol. 1963 Feb;85:369–381. doi: 10.1128/jb.85.2.369-381.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul K., Meyer A., Low M. G., Schachner M. Release of the 120 kDa component of the mouse neural cell adhesion molecule N-CAM from cell surfaces by phosphatidylinositol-specific phospholipase C. Neurosci Lett. 1986 Dec 23;72(3):341–346. doi: 10.1016/0304-3940(86)90538-0. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5793–5797. doi: 10.1073/pnas.83.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acid binding: a new kind of posttranslational modification of membrane proteins. Curr Top Microbiol Immunol. 1983;102:101–129. doi: 10.1007/978-3-642-68906-2_3. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Seki T., Moriuchi T., Chang H. C., Denome R., Silver J. Structural organization of the rat thy-1 gene. Nature. 1985 Feb 7;313(6002):485–487. doi: 10.1038/313485a0. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Action of phosphatidylinositol specific phospholipase C on platelets: nonlytic release of acetylcholinesterase, effect on thrombin and PAF induced aggregation. Life Sci. 1986 Feb 24;38(8):751–755. doi: 10.1016/0024-3205(86)90590-4. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Selective release of plasma-membrane enzymes from rat hepatocytes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1980 Apr 1;187(1):277–280. doi: 10.1042/bj1870277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D. Minireview. Phosphatidylinositol specific phospholipases C. Life Sci. 1982 Apr 19;30(16):1323–1335. doi: 10.1016/0024-3205(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. Characterization of the Phospholipases of Bacillus cereus and Their Effects on Erythrocytes, Bone, and Kidney Cells. J Bacteriol. 1965 Jul;90(1):69–81. doi: 10.1128/jb.90.1.69-81.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. MECHANISM OF ACTION OF THE TOXIN OF BACILLUS ANTHRACIS II. : Alkaline Phosphatasemia Produced by Culture Filtrates of Various Bacilli. J Bacteriol. 1962 Feb;83(2):359–369. doi: 10.1128/jb.83.2.359-369.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloski M. J., Uhr J. W., Vitetta E. S. Primary structural studies of the Qa-2 alloantigen: implications for the evolution of the MHC. Nature. 1982 Apr 22;296(5859):759–761. doi: 10.1038/296759a0. [DOI] [PubMed] [Google Scholar]
- Soloski M. J., Vernachio J., Einhorn G., Lattimore A. Qa gene expression: biosynthesis and secretion of Qa-2 molecules in activated T cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2949–2953. doi: 10.1073/pnas.83.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger A., Cardoso de Almeida M. L., Blatter M. C., Brodbeck U., Bordier C. The membrane-anchoring systems of vertebrate acetylcholinesterase and variant surface glycoproteins of African trypanosomes share a common antigenic determinant. FEBS Lett. 1986 Apr 21;199(2):182–186. doi: 10.1016/0014-5793(86)80476-8. [DOI] [PubMed] [Google Scholar]
- Stieger S., Brodbeck U. Amphiphilic detergent-soluble acetylcholinesterase from Torpedo marmorata: characterization and conversion by proteolysis to a hydrophilic form. J Neurochem. 1985 Jan;44(1):48–56. doi: 10.1111/j.1471-4159.1985.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Strang A. M., Williams J. M., Ferguson M. A., Holder A. A., Allen A. K. Trypanosoma brucei brucei variant surface glycoprotein contains non-N-acetylated glucosamine. Biochem J. 1986 Mar 1;234(2):481–484. doi: 10.1042/bj2340481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA K. R., VALENTINE W. N., FREDRICKS R. E. Diseases or clinical conditions associated with low leukocyte alkaline phosphatase. N Engl J Med. 1960 May 5;262:912–918. doi: 10.1056/NEJM196005052621804. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Asahi Y., Ikezawa H. Purification and properties of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Biochim Biophys Acta. 1980 Jul 14;619(1):48–57. [PubMed] [Google Scholar]
- Taguchi R., Ikezawa H. Phosphatidyl inositol-specific phospholipase C from Clostridium novyi type A. Arch Biochem Biophys. 1978 Feb;186(1):196–201. doi: 10.1016/0003-9861(78)90480-0. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Suzuki K., Nakabayashi T., Ikezawa H. Acetylcholinesterase release from mammalian erythrocytes by phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis and characterization of the released enzyme. J Biochem. 1984 Aug;96(2):437–446. doi: 10.1093/oxfordjournals.jbchem.a134855. [DOI] [PubMed] [Google Scholar]
- Takesue Y., Yokota K., Nishi Y., Taguchi R., Ikezawa H. Solubilization of trehalase from rabbit renal and intestinal brush-border membranes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1986 May 26;201(1):5–8. doi: 10.1016/0014-5793(86)80560-9. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Turner M. J. Antigenic variation in its biological context. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):27–40. doi: 10.1098/rstb.1984.0106. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Henthorn P. S., Lafferty M. A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Tse A. G. A glycophospholipid covalently attached to the C-terminus of the Thy-1 glycoprotein. Biosci Rep. 1985 Oct-Nov;5(10-11):999–1005. doi: 10.1007/BF01119912. [DOI] [PubMed] [Google Scholar]
- Woda B. A., Gilman S. C. Lateral mobility and capping of rat lymphocyte membrane proteins. Cell Biol Int Rep. 1983 Mar;7(3):203–209. doi: 10.1016/0309-1651(83)90227-8. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Yang J. C., Chang P. C., Fujitaki J. M., Chiu K. C., Smith R. A. Colvalent linkage of phospholipid to myelin basic protein: identification of phosphatidylinositol bisphosphate as the attached phospholipid. Biochemistry. 1986 May 6;25(9):2677–2681. doi: 10.1021/bi00357a058. [DOI] [PubMed] [Google Scholar]


