Abstract
Mental illnesses including anxiety disorders, autism spectrum disorder, post-traumatic stress disorder, schizophrenia, depression, and others exact an immense toll on the healthcare system and society at large. Depression alone impacts 21 million adults and costs over $200 billion annually in the United States. However, pharmaceutical strategies to treat mental illnesses are lagging behind drug development in many other disease areas. Because many of the shortcomings of therapeutics for mental illness relate to delivery problems, drug delivery technologies have the potential to radically improve the effectiveness of therapeutics for these diseases. This review describes the current pharmacotherapeutic approaches to treating mental illnesses as well as drug delivery approaches that have improved existing therapies. Approaches to improve drug bioavailability, provide controlled release of therapeutics, and enable drug targeting to the central nervous system (CNS) will be highlighted. Moreover, next-generation delivery approaches such as environmentally-controlled release and interval/sequential drug release will be addressed. Based on the evolving landscape of the treatment of mental illnesses, the nascent field of drug delivery in mental health has tremendous potential for growth in terms of both economic and patient impact.
1. Introduction
1.1. Overview and Impact of Selected Mental Illnesses
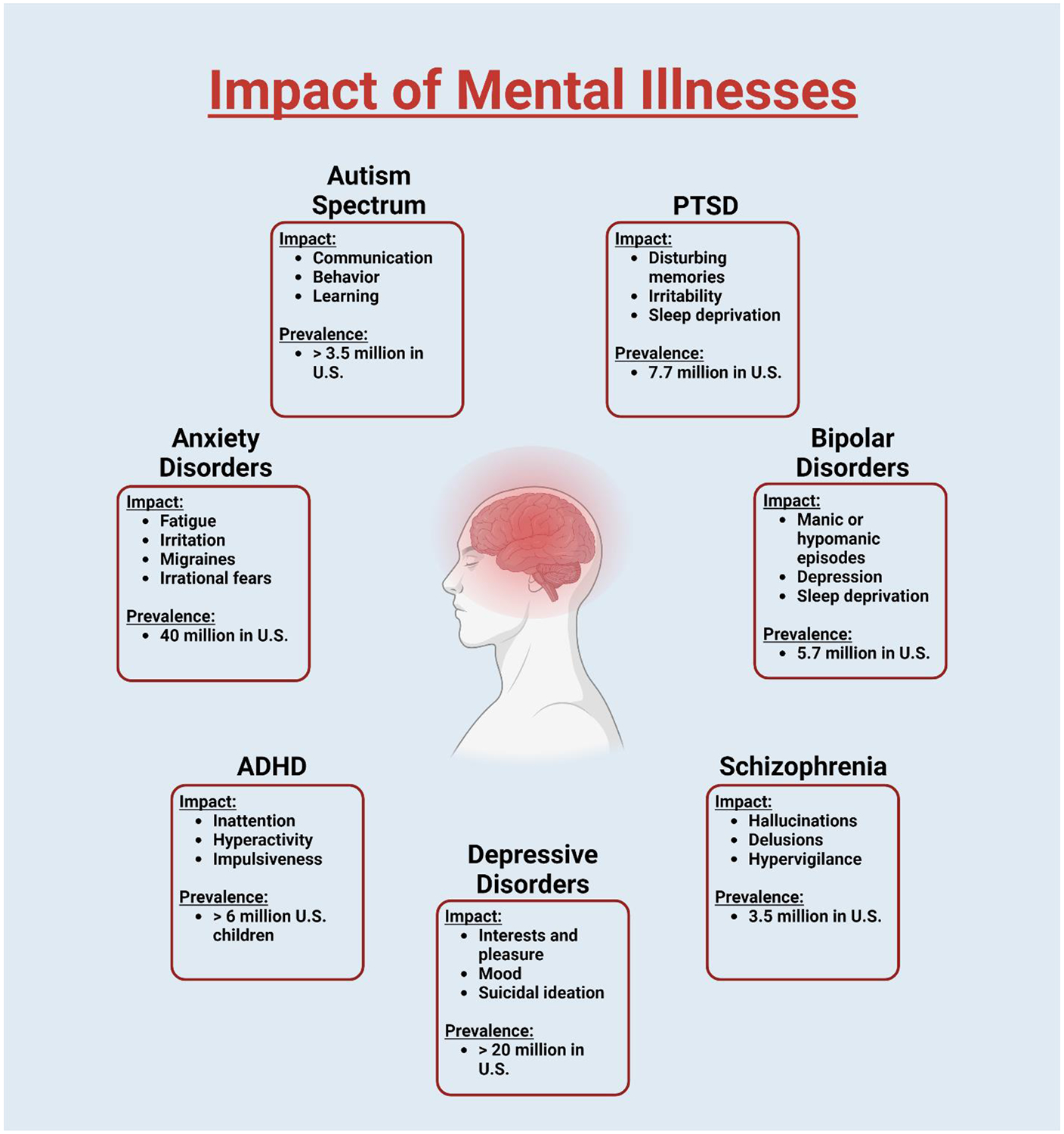
The science behind creating effective drug delivery mechanisms is always challenging and multifaceted, but perhaps even more nuanced when it comes to psychiatric medicine. The brain is an anatomically privileged organ with many delivery barriers that make it exceptionally difficult to target. Patients suffering from mental illnesses are some of the most likely to struggle with drug compliance and issues of abuse and diversion. Different mental illnesses present different factors that contribute to diminished compliance. Depressive patients might struggle to motivate themselves to follow dosing schedules, whereas schizophrenic patients might struggle to accept the medications for what they are or may believe they do not need medication at all. Novel delivery methods could drastically improve compliance by removing scheduling or self-administration issues via prolonged drug delivery, or by simply increasing the comfort or reducing the pain associated with some traditional administration methods. However, the lack of a complete understanding of the pathophysiology of many disorders in this space makes it difficult to design effective pharmacotherapies. This review serves to introduce the more prevalent mental illnesses affecting society today, overview their current treatment options, and highlight the latest research and future directions of drug delivery approaches that could improve the treatment of mental illness. Disorders selected for the discussion include anxiety disorders, autism spectrum disorder, post-traumatic stress disorder, bipolar disorders, schizophrenia, major depressive disorder, and attention-deficit/hyperactivity disorder (Figure 1).
Figure 1. Impact and prevalence of mental illnesses discussed in this article.
1.2. Anxiety Disorders
Intermittent anxiety is an inevitable and occasionally necessary aspect of life. Whether that anxiety arises from family crises, job/school pressure, or health-related issues, healthy individuals will experience anxiety throughout their lives. However, once that anxiety weaves itself into all aspects of living, intermittent anxiety becomes a debilitating anxiety disorder. The National Institute of Health (NIH) categorizes anxiety disorders into panic disorder (PD), social anxiety disorder (SAD), generalized anxiety disorder (GAD), and various phobia-related disorders. These patients may experience certain symptoms that include but are not limited to restlessness, fatigue, mental irritation, sudden and inexplicable pain (headaches, muscle pain, stomachaches, etc.), insomnia, sweating, chest pain, trembling, difficulty in making eye contact with people, and/or irrational fear over specific objects or circumstances.1-7 With a global prevalence of 7.3% [4.8%-10.9%], anxiety disorders are often thought to be the most prevalent psychiatric disorders.8 Among anxiety disorders, specific phobias are the most common, followed by panic disorder, social phobia, and then generalized anxiety disorder. The prevalence of these disorders has undoubtedly risen in the last century, but researchers are unclear as to whether this is a result of more readily accessible resources and a heightened comfort in self-reporting, or if this increased prevalence is a real phenomenon arising from physiological and/or societal influence (changes in agriculture, pharmaceuticals, societal values, etc.). Women are more prone to develop anxiety disorders than men (1.5 to 2 times more likely), and often with an onset at adolescence.9,10 Again, it is unclear whether this discrepancy is the result of a true difference between the two physiologically, or simply a statistical error arising from societal pressure on men to suppress emotional vulnerability. There is a significant comorbidity between anxiety (especially GAD or PD) and depressive disorders. Additionally, anxiety disorders often have a marked overlap of symptoms, which makes treatment even more difficult for non-specialist physicians. As a result, anxiety disorders are tragically undertreated, underdiagnosed, and/or misdiagnosed in primary care.11
1.3. Autism Spectrum Disorder
Autism, also referred to as autism spectrum disorder (ASD), is a set of clinical phenotypes thought to induce the onset of neurodevelopmental issues that can affect social communication, imagination, and behavior.12 Other disorders (e.g., developmental coordination disorder (DCD), tic disorders, communication disorders, intellectual disability (ID), and attention-deficit/hyperactivity disorder (ADHD)) imbricate significantly with autism: a misfortune that can make it challenging to separate these disorders from one another, and even more so at the earlier stages of development.13-17 This grouping of similarly presenting disorders has increasingly been referred to as ESSENCE (early symptomatic syndromes eliciting neurodevelopmental clinical examinations).18
It has historically been challenging for researchers to identify any specific pathognomonic symptom of autism. Instead, an emphasis has been put on portraying and mapping the “autism gestalt”; these characterizations have been published in several operationalized symptom criteria sets (American Psychiatric Association, 1980, 1987, 1994, 2012).18 Most of these theories have been built on Wing’s suggested triad of impairments (social, communication, and imagination).19 The current criteria used to diagnose ASD can be found in the Diagnostic and Statistical Manual of Mental Disorders (DSM) published by the American Psychiatric Association (APA).20 The pervasive developmental disorder criteria (DSM-IV) and autism spectrum (DSM-V) for autistic disorder have been around for some 30 and 10 years, respectively, and are widely considered “standardized” for the gestalt of autism.21 The Gillberg and Gillberg criteria for Asperger’s syndrome have been implemented for some 25 years and are widespread in clinical use for patients presenting with an “autism phenotype”.22 Boys are believed to express symptoms of autism at higher rates and levels than girls, with male-to-female ratios of 3–5:1 being reported.23 That having been said, certain studies have recently suggested that females with slightly varying phenotypes may still have the social communication disorders that are typical of males with a formal diagnosis of autism, and so the “real” male-to-female ratio could be much closer to the 1:2 ratio often cited for other neurodevelopmental disorders.24
1.4. Post-Traumatic Stress Disorder
Post-traumatic stress disorder (PTSD) is a condition that most often arises as a result of being exposed to severely traumatic events such as combat, natural disasters, interpersonal violence, the loss of a loved one, and/or life-threatening accidents.25 Though the symptoms of PTSD are often quickly identified by a physician, the substantial overlap between the symptoms of PTSD, depression, and other anxiety disorders creates conditions in which the proper diagnosis is easily missed. Those misdiagnoses can largely be avoided if certain inquiries are made regarding the experiencing of a traumatic event, but unfortunately, some PTSD patients so heavily repress these associated memories that even a proper inquiry may yield the wrong diagnosis.26 Symptoms of PTSD include trauma-related nightmares, distressing or disturbing memories, irritability, hypervigilance (heightened threat sensitivity or constant anxiety over the potential for danger), sleep deprivation, reduced ability to concentrate, and/or emotional withdrawal.25 Individuals with PTSD often strive to avoid activities, places, items, and even people that could remind them of their trauma. As is the case with many mental disorders, the severity of PTSD is heightened by co-occurring conditions that develop in parallel with, or directly as a result of, symptoms arising from PTSD.27 Often associated co-occurring conditions include mood and anxiety disorders, impulsive behavior, self-destructive behavior, and/or substance abuse.28 Additionally, PTSD has been found to have significant comorbidities (inflammation, chronic pain, cardiometabolic disorders, and an increased chance of developing dementia).28,29 As a result, the severity of the total disease burden, or the premature mortality plus the general disability, is extremely high in PTSD patients.28 In order to be diagnosed with PTSD, a person has to have been exposed to some significant stressor or traumatic event to which he or she responded with helplessness or fear (again, occasionally challenging to navigate given the tendencies of some patients to repress such memories). Beyond those baseline requisites, the patient should have at least one symptom associated with, or falling under the umbrella of, each of these three definable classes: reexperiencing the trauma, avoiding reminders of the trauma, and experiencing hyperarousal (worsening concentration and irritability, increasing hypervigilance, and increasing unmerited reactions to traditionally non-arousing events) for at least one month.30
1.5. Bipolar Disorders
Before its labeling or characterization in the 1850s by Falret, bipolar disorder was considered to be directly under the umbrella of depressive disorders.31 Bipolar disorder is still a form of a mood disorder but is now acknowledged as a separate category of mental disorders encompassing bipolar I, bipolar II, and cyclothymic disorders.32 The distinct trait that separates bipolar disorders from other depressive disorders is the presence of cyclic manic or hypomanic episodes that may alternate with depressive episodes.33 Patients with bipolar I disorder experience apparent manic episodes with a varying array of manifestations, including flamboyance, overconfidence, talkativeness, excessive self-consciousness, irritability, sleeplessness, and highly elevated responses to minor mishaps.34 Hallucinations and delusions occur in up to 75% of manic episodes, and even those episodes lacking hallucinations and delusions may damage psychosocial functioning so severely that the patient requires hospitalization.35 Patients with bipolar II disorder differ from those with bipolar I in that they experience periods of depression coupled with hypomania as opposed to mania.35 Just a singular instance of a hypomanic episode, for most physicians, is the only requisite for the diagnosis of bipolar II disorder.35 Cyclothymic disorder, the third disorder falling under the bipolar umbrella, is marked by cyclic periods of depression and hypomania, lasting for a minimum of two years, that do not align with the diagnostic criteria for a major affective episode. During instances of increased mood, patients with a bipolar disorder may ironically be affected even more so by depressive symptoms.35 The onset of bipolar disorder typically presents itself to patients approximately 20 years of age. Oftentimes, the earlier the onset, the worse the prognosis (more debilitating depressive episodes, higher levels of anxiety, and increased risk of substance abuse).36 Usually, depressive episodes are the first to present in patients with bipolar, and for the majority of patients with bipolar I or bipolar II disorder, the duration of depressive episodes is notably longer than that of manic or hypomanic episodes – a major reason that bipolar disorders are often misdiagnosed as major depressive disorder.37 The World Mental Health Survey Initiative estimated 12-month and lifetime prevalence for bipolar disorders to be 1.5% and 2.4%, respectively.38 As is the case with most psychological disorders, prevalence rates greatly vary by country: a difference arising mostly as a result of methodologic and cultural differences.38
1.6. Schizophrenia
Schizophrenia, arguably the most complicated psychiatric disorder, can be typified by an array of symptoms such as hallucinations, delusions, muddled speech, unrealistic behavior, diminished emotional expressions, and blighted cognitive ability.39 Positive symptoms, those symptoms like hallucinations and delusions that introduce some new psychological experience, are often the pushing factor for a patient to seek the guidance of a physician. However, the illness is frequently accompanied by negative symptoms (symptoms that remove pre-existing positive psychological states) such as demotivation, social withdrawal, and cognitive symptoms: symptoms that potentially include memory loss and/or lessened executive function and processing speed.40 Though more recent connotations and descriptions accentuate positive symptoms, earlier conceptualizations considered negative symptoms as core attributes of the disorder.41 It is largely agreed upon that the negative symptoms greatly contribute to the long-term burden experienced by schizophrenic patients.42 The disorder usually becomes apparent in early adulthood where an often well-defined prodromal period precedes the appearance of the first psychotic episode.40 Though the prevalence of schizophrenia is less than 1%, the associated frequent healthcare visits, relatively high inpatient needs, exploratory nature of medicating, and negative impact on job retention all work together to create a relatively extensive healthcare burden, with yearly associated costs in the United States estimated to be more than $150 billion.43,44 Patients with schizophrenia tend to have reduced life expectancy (some 15 fewer years on average) and a more substantial risk of suicide than the general public.45-47
1.7. Major Depressive Disorder
Major depressive disorder (MDD) is diagnosed after clearcut changes in mood, interests, pleasure, and cognitive function have persisted for at least two weeks; these symptoms and duration are frequently referred to as discrete depressive episodes.48 With how closely the symptoms associated with a major depressive episode and MDD overlap with the depressive symptoms seen in schizophrenia and bipolar disorder, the implementation of a well-defined exclusion criterion is required for a proper diagnosis of MDD.48 Literature shows that women are about twice as prone to develop MDD compared to men and that roughly 6% of the global adult population experiences MDD each year.49,50 When measured by the years lived with a disability, MDD is the second leading medical condition: a statistic that well highlights the individual and public burden of this chronic disease. Beyond the commonly associated symptoms, MDD has been shown to increase the risk of developing heart disease, diabetes mellitus, stroke, and other conditions.51 Furthermore, it is estimated that some 50% of the 800,000 suicides per year take place during a depressive episode and that patients with MDD, when compared to the general population, are roughly 20 times more likely to die by suicide.52 A study observing the contribution of genetics to MDD showed that genetic variables (mostly rare mutations) do in fact play some non-negligible role in the risk of MDD development.53 Additionally, childhood abuse (sexual, physical, or emotional), as well as other environmental factors, have been shown to heighten the risk of developing MDD.54 That having been said, the current understanding of how these factors interact with one another is exceedingly lacking.53 Changes in the neurobiological networks that regulate the stress response (autonomic nervous system, immune system, and the hypothalamic-pituitary-adrenal axis) have and can be observed in patients with MDD.55 A recent study reported that, in the United States, the percentage of adults with MDD went up 12.9% (15.5 to 17.5 million) between 2010 and 2018.56 Over the same period, the United States witnessed a 37.9% increase in the MDD-associated economic burden (236.6 billion to 326.2 billion USD).56
Up to 50% of persons with diagnosed MDD either do not respond or respond poorly to existing therapies; these patients are considered treatment-resistant.57,58 Even though treatment-resistant depression (TRD) patients are prescribed higher doses of benzodiazepines and antipsychotics, they continue to experience longer depressive episodes with higher rates of severe depression, suicide attempts, and hospitalizations.59 Job loss and financial stress are more prevalent among TRD patients, and these patients incur an estimated $29-48 billion increase in healthcare expenses compared to treatment-responsive MDD patients.60,61 Non-pharmaceutical medical interventions are available for TRD patients but are often undesirable due to side effects, costs, limited availability, and/or lack of efficacy. For example, electroconvulsive therapy, while effective, causes retrograde amnesia and repetitive transcranial magnetic stimulation, requiring travel to a clinic for treatment five days per week.62
1.8. Attention-Deficit/Hyperactivity Disorder
Attention-deficit/hyperactivity disorder (ADHD) is a neurobehavioral disorder typified by persistent symptoms of inattention, hyperactivity, and impulsiveness which affects an estimated 2–4% of adults according to the DSM-V.63,64 Attributes of ADHD start to develop during childhood, and the estimated prevalence rate of 5%-8% in children (5-18 years old) suggests that symptoms or attributes frequently endure into adulthood, though it is not yet clear why the rates drop into adulthood (i.e., is there a substantive drop or is there a generational gap in diagnosis?).65 ADHD is considered to be a significantly heterogenous (clinically) disorder: a result of the high rates of comorbidity with other childhood-onset disorders. It is reported that the vast majority (60%-100%) of juvenile ADHD patients also display one or more psychiatric and social comorbidities that often continue into the later stages of their lives.66,67 For example, as patients with ADHD mature into adulthood, their struggles with heightened risks of impairments in education and academic performance can lead to criminal behavior and physical or mental health problems.68,69 Though it is fairly well accepted that ADHD is overall more common in boys than in girls, there are conflicting opinions on the prevalence by gender in adults.70 Some studies suggest a higher prevalence of men with ADHD, and yet, others show equivalent or even higher prevalence in women.71,72
2. Current Pharmacotherapeutic Approaches
2.1. Introduction and Drug Overview
Pharmacotherapies have been an important approach to treat mental illnesses, and although much room for improved therapy still exists, these treatments have provided marked improvements in mental health therapy over the last several decades.11 Moreover, a meta-analysis based on 234 randomized controlled studies published in 2015 infers that, on average, pharmaceuticals induced a much higher effect size (Cohen’s d=2.02) than psychotherapies (d=1.22; P < 0.0001).73 Additionally, the article showed that a higher effect size (d=2.12) could be achieved by combination approaches (psychotherapy with pharmacotherapy). Food and Drug Administration (FDA)-approved drugs and drug classes to treat mental illnesses are summarized in Table 1.
Table 1.
Summary of mental illnesses, FDA-approved drug classes, and specific drugs approved for the treatment of each mental illness.
| Illness | FDA-Approved Drug Type/Class |
Generic Names | References |
|---|---|---|---|
| Anxiety Disorders | SSRIs | Fluoxetine, Sertraline, Citalopram, Escitalopram, Paroxetine, Paroxetine ER, Fluvoxamine | 74 |
| SNRIs | Duloxetine, Venlafaxine (XR), Desvenlafaxine | ||
| TCAs | Clomipramine, Imipramine, Desipramine, Nortriptyline | ||
| MAOIs | Phenelzine | ||
| Benzodiazepines | Alprazolam, Lorazepam, Chlordiazepoxide, Oxazepam | ||
| Antipsychotics | Trifluoperazine | ||
| Antihistamines | Hydroxyzine | ||
| Autism Spectrum Disorder (ASD) | Antipsychotics | Risperidone, Aripiprazole | 75 |
| Post-Traumatic Stress Disorder (PTSD) | SSRIs | Sertraline (Zoloft), Paroxetine (Paxil), Fluoxetine (Prozac) | 76 |
| Antidepressants | Venlafaxine (Effexor) | ||
| Bipolar Disorders | Salts | Lithium | 77 |
| Atypical Antipsychotics | Aripiprazole, Asenapine, Cariprazine, Lurasidone, Olanzapine, Olanzapine/fluoxetine combination, Quetiapine, Risperidone, Ziprasidone | ||
| Anticonvulsants | Carbamazepine, Lamotrigine, Valproate, Lamotrigine | ||
| Schizophrenia | First-generation Antipsychotics | Chlorpromazine, Haloperidol, Loxapine, Perphenazine, Prochlorperazine, Thiothixene, Thioridazine, Trifluoperazine | 78 |
| Second-generation Antipsychotics | Aripiprazole, Asenapine, Clozapine, Iloperidone, Olanzapine, Paliperidone, Quetiapine, Risperidone, Ziprasidone | ||
| Major Depressive Disorder (MDD) | TCAs | Trimipramine (Surmontil), Amoxapine, Clomipramine, Desipramine (Norpramin), Imipramine (Tofranil), Maprotiline, Nortriptyline, Nortriptyline (Pamelor), Protriptyline (Vivactil), Amitriptyline, Doxepin (Sinequan) | 79 |
| MAOIs | Isocarboxazid (Marplan), Phenelzine (Nardil), Selegiline (transdermal patch), Tranylcypromine (Parnate) | ||
| SSRIs | Citalopram (Celexa), Escitalopram (Lexapro), Fluoxetine (Prozac), Fluvoxamine, Paroxetine (Paxil, Pexeva), Sertraline (Zoloft) | ||
| SNRIs | Desvenlafaxine (Pristiq), Duloxetine (Cymbalta), Venlafaxine (Effexor) | ||
| DRIs | Bupropion (Wellbutrin) | ||
| TCAs | Mirtazapine | ||
| Attention Deficit/Hyperactivity Disorder (ADHD) | CNS Dopamine Stimulants | Methylphenidate, Amphetamine | 80 |
| Non-stimulants | Atomoxetine, Guanfacine, Clonidine, antipsychotics, tricyclic antidepressants, bupropion, modafinil, venlafaxine, or a combination |
Abbreviations: SSRI- Selective Serotonin Reuptake Inhibitors; SNRI – Serotonin Norepinephrine Reuptake Inhibitors; TCA - Tricyclic Antidepressants; MAOI – Monoamine Oxidase Inhibitors; DRI - Dietary Reference Intakes; CNS- Central Nervous System.
2.2. Anxiety Disorders
FDA-approved medication classes for diseases falling under the umbrella of anxiety disorders include benzodiazepines, antipsychotics, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and antihistamines. Given their benefit/risk balancing capabilities, SSRIs and SNRIs are the current gold standard.73 SSRIs and SNRIs are both antidepressants but with different mechanisms of action. SSRIs work by increasing serotonin levels in the brain; SNRIs work by increasing both serotonin and norepinephrine levels. Another medication that reduces the effects of anxiety disorder is benzodiazepine – a drug that alters the neuronal activities triggering anxiety. However, given their potential side effects such as high mortality rate, risk of addiction, increased risk of dementia, and escalated suicide risk, benzodiazepines are not currently recommended as a primary treatment, but should a drug delivery method be able to minimize those risks, benzodiazepines could prove to be a more accessible pharmacotherapy.81
2.3. Autism Spectrum Disorder
For autism spectrum disorder (ASD), psychotherapeutic treatments and lifestyle changes are the recommended first-line interventions, particularly for patients under seven or eight years of age.82 Those psychotherapeutic treatments used include schema therapy, cognitive behavior therapy, narrative therapy, and positive behavior support.83 Additionally, when needed, occupational therapists can assist with improving shortcomings in motor skills, interception skills, and social skills.83 Circumstantially, pharmaceuticals could enhance the treatment plan when prescribed by a psychiatrist; those drugs generally aim to improve the non-pharmaceutical interventions and decrease the risk factors of comorbidities. The most common pharmaceutical agents used for ASD are antipsychotics.82 The major consideration for antipsychotic treatment is to start with a low initial dose and then slowly increase it to achieve the greatest therapeutic effect with the lowest dose. This is of course the case with most medications, but even more so with antipsychotics. With the treatment of autism spectrum disorder, doctors must be cognizant of the risks and increased likelihood of polypharmacy.84 However, persons with ASD frequently exhibit symptoms that indicate alterations in different systems of the brain; this can make it exceedingly difficult to employ just one drug for the treatment of all presenting symptoms.84 The two currently FDA-approved products for the irritability associated with autism include risperidone and aripiprazole. Multiple studies and clinical trials have shown that antipsychotics risperidone and aripiprazole are capable of decreasing irritability, anxiety, lethargy, social withdrawal, stereotypical attitude, hyperactivity, and inappropriate speech: all common syndromes of ASD.85
2.4. Post-Traumatic Stress Disorder
Highly specialized strategies are often required for the treatment of PTSD. Those strategies should allow and encourage the patients to control their fears and emotional responses to their traumas. Therapeutic treatment often aims at reducing the degree and frequency of torment that arises from memories of the traumatic event and suppressing the physiological symptoms that may arise from that stress.25 Various research efforts have highlighted the efficacy of a number of techniques from exposure therapy (encouraging the confrontation of painful feelings/memories), to cognitive therapy (encouraging the formal arrangement and characterization of thoughts and beliefs), to anxiety management, and even interpersonal therapies (encouraging the acknowledgment that the trauma continues to stress relationships and social wellbeing).86 Certain medications have also been reported to be helpful for traumatized patients. Results of randomized clinical trials demonstrate that antidepressants (SSRIs, tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs)) improve the overall functioning of patients struggling with PTSD.87 Serotonin-reuptake inhibitors are the current gold standard because they are less symptomatic than other types of psychotropic medications. SSRIs e.g., sertraline (Zoloft) and, more recently, paroxetine (Paxil) are still the only FDA-approved drugs for the treatment of PTSD.88
2.5. Bipolar Disorders
Pharmacotherapies with mood stabilizers and/or antipsychotics are the current gold standard for the treatment of hypomania and mania in bipolar disorder patients. Hypomania-specific drugs are limited, so therapies for mania are frequently also used for hypomania.35 Network meta-analyses have suggested that between three antipsychotics (risperidone, aripiprazole, and valproate), risperidone was the most effective.89 If there is little to no response to the therapy after 1 to 2 weeks, the drug or drug combination may need to change. Particularly for severe mania, the combination of antipsychotic(s) and mood stabilizer(s) is believed to be more effective than either medication by itself. During episodes or periods of depression, patients often have more ‘unacceptable’ side effects from their medicine than they do during a manic episode. A few small trials found support for the implementation of mood stabilizers (pramipexole, ketamine, and scopolamine) for the treatment of symptoms arising from episodes of bipolar depression.90 Additional secondary treatment with agents such as anti-inflammatory drugs, N-acetylcysteine, n–3 polyunsaturated fatty acids, and pioglitazone has been reported as having antidepressant effects in persons with bipolar depression.91 Those claims have yet to be supported by trials with large sample sizes.90,91 A relatively recent meta-analysis proposed that second-generation antidepressants (e.g., SSRIs and norepinephrine/dopamine reuptake inhibitors (NDRIs)) could be valuable for the short-term management of bipolar depression.92 There are currently only three drug types that are FDA-approved for the treatment of bipolar disorders: lithium salt, atypical antipsychotics, and anticonvulsants.
2.6. Schizophrenia
Several first and second-generation antipsychotics are FDA-approved for the treatment of schizophrenia. Clozapine, the first atypical, second-generation antipsychotic, has proven more effective in the treatment of schizophrenia as compared to first-generation antipsychotics.93 Relevant to the rest of this discussion, clozapine, given its sensitivity to hepatic first-pass metabolism, has a bioavailability of just 27% when administered orally.93 To resolve this issue, a transdermal patch formulation was developed so that the biotransformation of clozapine could be bypassed entirely, hence enhancing its bioavailability, as will be discussed further in Section 3 of this review. Atypical antipsychotics, or second-generation antipsychotics (SGAs), act on various receptors with a high affinity to the serotonin 2A receptor (5HT2A) and less affinity to the D2 receptor leading to a great reduction in extrapyramidal side effects.94
2.7. Major Depressive Disorder
Traditional medications for major depressive disorder include SSRIs, SNRIs, atypical antidepressants, TCAs, and MAOIs.95,96 The use of antidepressants for treating MDD began in the late 80s with the introduction of fluoxetine which, like other SSRIs to follow, became wildly popular due to its relatively few side effects, overdose safety, and ease of use when compared with previously available medications, such as TCAs and MAOIs.97 In addition to the SSRIs, several SNRIs are commonly prescribed. The goal of prescribing SNRIs is to produce an activity profile similar to that of the TCAs while also providing a wide therapeutic index.96 Several other medications have been introduced, but like the SSRIs and SNRIs, as well as the prior generation of medications, these agents act primarily through the modulation of serotonin and norepinephrine transport. Emerging and investigational antidepressants closest to clinical practice largely continue to focus on the modulation of monoamine neurotransmission. However, all these current protocols for MDD are not fast-acting on the patients as the increase in serotonin at the axon terminals usually weeks to provide therapeutic benefit.98 Additionally, about half of the patients suffering from MDD do not show any signs of improvement with their current medications; these patients are considered treatment-resistant.99
In addition, classical psychedelics have re-emerged as a promising pharmacotherapy for major depressive disorder (MDD) by reducing many of the issues commonly associated with traditional antidepressants. Classical psychedelics span several chemical classes, but they all have activity at the serotonin 2A receptor (5HT2A). In 2016, the first groundbreaking clinical trial analyzing the value of psychedelics for treating depression was published by Carhartt-Harris and colleagues,100 with a secondary analysis in 2018.101 20 patients with moderate-to-severe unipolar treatment-resistant depression (TRD) were given two oral doses of psilocybin. Psychological support was provided before, during, and after each session. Even with the conservative response criterion used in this study, 9 patients were responders (45%) with six of these nine maintaining response at six months (30%). An acute change in plasticity caused by psychedelic-induced 5HT2A receptor signaling was the mechanism proposed by the authors.102-105 A more recent study by Daws et al. reported two clinical trials where psilocybin was administered to 19 TRD patients to evaluate its subacute impact on brain function (Figure 2).106 Trial one was an open-label trial of orally administered psilocybin (10 mg and 25 mg, 7 d apart) whereas the second trial was a double-blind phase II randomized controlled trial comparing psilocybin therapy with escitalopram. In both trials, the antidepressant response to psilocybin was rapid, sustained, and correlated with decreases in fMRI brain network modularity, implying that psilocybin’s antidepressant action may depend on a global increase in brain network integration.106 The clinical trial data show that the 5HT2A receptor-rich higher-order functional networks became more functionally interconnected and flexible after psilocybin treatment.106
Figure 2. Psilocybin for rapid, sustained alleviation of treatment-resistant depression (TRD).
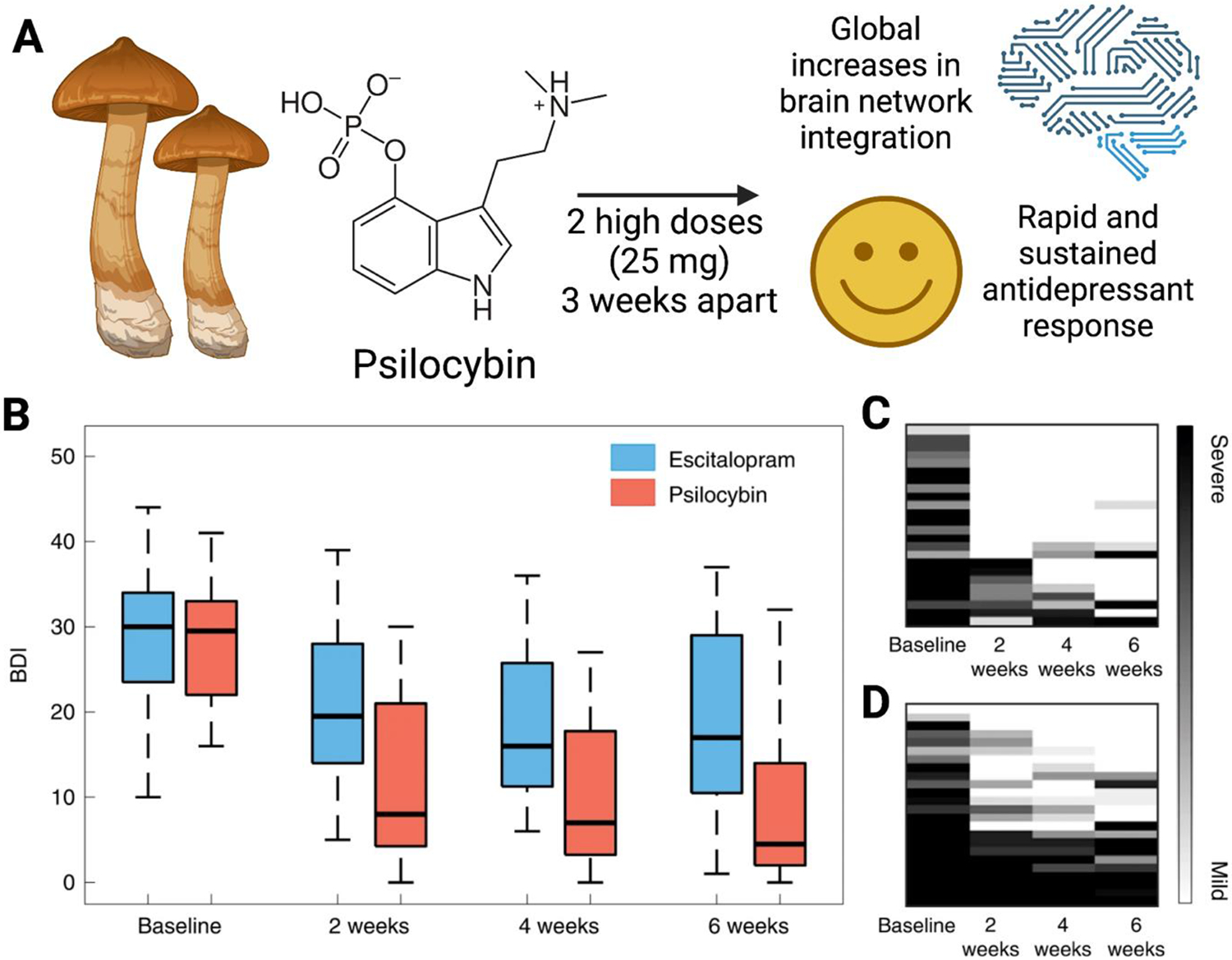
(A) High-dose psilocybin leads to increases in brain network integration and antidepressant effects. (B) Beck depression inventory (BDI) scores from each study arm (psilocybin vs. escitalopram) and time point. Qualitative raster plots of individual patients’ BDI for each time point for the psilocybin arm (C) and escitalopram arm (D). Adapted from Reference 106.
These results, among others, moved the FDA to grant “Breakthrough Therapy” designation to psilocybin, a 5HT2A agonist and Schedule I controlled substance, to treat TRD. In further support of the notion that psychedelics can improve TRD outcomes, Nichols et al. recently reported persistent antidepressant-like effects of psilocybin and lysergic acid diethylamide (LSD) that exceeded the efficacy of ketamine in rodent models.107 Thus, their unique mechanism of action and early demonstrations of safety and efficacy highlight the promise of 5HT2A agonists as novel pharmacotherapies for TRD.
2.8. Attention-Deficit/Hyperactivity Disorder
Pharmacological treatments are the most effective and widely used first-line treatments for ADHD.108 However, predominantly as a result of adverse effects and limited effectiveness, about 50% of patients stop taking prescribed drugs within the first 3 years of treatment.109 A few recent studies showed that cognitive-behavior-based treatments could be effective for adults with ADHD.110 Other types of non-pharmacological approaches need to be studied to understand their impact on secondary symptoms of ADHD (social functioning).110 There is a growing list of FDA-approved medications for ADHD. Behavioral approaches from family and school along with pharmacologic methods have been proven to be the most effective treatments for ADHD.111 Both stimulants (methylphenidate or amphetamines) and non-stimulants (norepinephrine reuptake inhibitor, atomoxetine, α-2 adrenergic agonists, extended-release guanfacine, extended-release clonidine, antipsychotics, TCAs, bupropion, modafinil, venlafaxine, or a combination) have been used for the pharmacological treatment of ADHD.111-113 The effect size for stimulants is 1.0 and for non-stimulants is 0.7, therefore, for most adolescents, stimulant medications are highly effective in reducing ADHD’s core symptoms.112 Stimulants, the most commonly prescribed medication for ADHD, increase dopamine levels in the brain.114 Dopamine is a neurotransmitter associated with motivation, pleasure, attention, and movement.114 For many people with ADHD, stimulant medications boost concentration and focus while reducing hyperactive and impulsive behaviors.114 Non-stimulants are more recent medications for ADHD; they target norepinephrine instead of dopamine and are often prescribed with stimulants.115 The use of non-stimulants may result in fatigue, dizziness, rash, and/or increased blood pressure, whereas stimulants have fewer side effects in comparison.116
3. Approaches to Drug Delivery in Psychiatry
3.1. Transdermal Delivery
Though introduced and then popularized in the 60s and 70s, transdermal delivery has only recently been applied to psychiatry. Transdermal delivery could help psychiatric patients by offering reduced dosing frequencies, the avoidance of painful or stressful parenteral administration, more effective concentration control, improved tolerability, more direct compliance confirmation, and the avoidance of first-pass hepatic metabolism. Transdermal delivery technologies are often categorized by the generation or time at which they were introduced or created.117 First-generation transdermal delivery systems make up the majority of all currently FDA-approved transdermal drugs. These delivery systems, similar to typical topical medications such as ointments, do not provide permeability enhancement. This generation requires the drug of choice to be low-molecular-weight, lipophilic, and efficacious at small doses. Though simple and perhaps limited in many ways, these systems can still be useful by providing a pathway towards less frequent dosing, more steady delivery profiles, and better patient compliance for those patients that struggle with pill ingestion. The second-generation systems are those that address issues with skin permeability. These systems attempt to increase skin permeability by disrupting the stratum corneum, providing a driving force for transport, and avoiding injury to the underlying tissue. With these second-generation approaches (iontophoresis and non-cavitational ultrasound), making meaningful improvements in delivery across the stratum corneum often leads to damage to the deeper tissues. This setback is ultimately what led to the creation of third-generation transdermal delivery systems. These systems aim for a stronger disruption of the stratum corneum, whereby drugs can be more effectively delivered through the outer layer of the skin while preserving the deeper tissues such as the dermal and epidermal layers. Methods to achieve this targeting include chemical enhancers (e.g., sodium laureth sulfate and phenyl piperazine), biochemical enhancers (e.g., synthetic peptides), electroporation, microneedles, and thermal ablation. Equally as important, this generation of systems has made it possible to deliver macromolecules (e.g., therapeutic proteins and vaccines).
A study from 2002 conducted a double-blind, placebo-controlled, parallel-group study in outpatients with major depression by comparing the effects of a selegiline transdermal system against a placebo, where selegiline ((R)-(−)N,2-dimethyl-N-2-propynylphenethylamine HCl) is a highly selective, irreversible MAO-B inhibitor at low doses. This 6-week study had a sample size of 176 (N=89 for DOI, N=88 for placebo) and observed a 46% greater improvement on the 17-item Hamilton depression scale, a 52% increase on the 28-item Hamilton depression scale, and a 79% improvement on the Montgomery-Asberg depression rating scale. 118 Another study, carried out in 2020, presented a case series where six patients with TRD were simultaneously treated with ketamine (intravenously) and selegiline (transdermally). Though the sample size was small, they found convincing evidence that combination therapies could prove to be extremely valuable in the treatment of TRD. In this case, they believe that IV ketamine with transdermal selegiline could yield significant improvements for TRD patients not able to undergo electroconvulsive therapy. 119 Transdermal selegiline, when compared to oral selegiline, was found to offer more steady and prolonged plasma concentrations, negligible peak-through fluctuations, increased bioavailability, and reduced concentrations of metabolites. The clinical use of MAOIs was declining over concerns with their poor food and drug interactions. Transdermal selegiline was shown to combat this poor interaction by inhibiting brain MAO-A and MAO-B enzymes with minimal effect on gastrointestinal MAO-A – a novel inhibition mechanism that greatly reduced the risk of interactions with tyramine-rich foods. Transdermal selegiline is increasingly gaining interest because it has been shown to have implications for not just depression, but also PTSD and anxiety disorders. A small study observed the positive effects of transdermal selegiline on symptoms of social phobia, but more studies are needed. 120,121
Nicotine has been another drug of interest in the transdermal delivery space. Most notably, researchers have been looking into the effect of transdermal nicotine on depression and autism. A small-scale, double-blind, placebo-controlled trial analyzed nicotine and its effect on the attenuation of depression in nonsmokers. This study found that an eight-day long program consisting of 3.5 milligrams a day of transdermal nicotine decreased depression symptoms (CES-D) and improved response inhibition (Conners CPT). The small-scale nature of this study discourages any confident conclusions regarding the effects of transdermal nicotine, but the findings were certainly significant enough to merit further research. 122 A case was reported where transdermal nicotine was used for the treatment of a hospitalized adolescent with autism spectrum disorder. This decision was based on pre-existing research that suggested nicotine administration could significantly reduce aggression-related behaviors. Given that the nicotine patch used in the study was recurrently well-tolerated and clearly reduced the need for emergency medication or restraint, the conductors of this study believe that the results witnessed were enough to merit a larger study on the effects of transdermal nicotine administration on the dampening of aggressive behaviors arising from autism. 123
Also of interest, transdermal clonidine has shown implications in the improvement of anxiety, ADHD, and autism. A study performed in 2018 described a patient who had historically been unresponsive to traditional approaches (SSRI, mood stabilizing drugs, and antipsychotics), but once treated with transdermal clonidine, experienced a significant clinical outcome. In further support of clonidine, a systematic literature review carried out in 2020 looked at the effect of treating behavioral disturbances arising from autism spectrum disorder with clonidine and found that there has been enough evidence to support the use of clonidine for ADHD, and they go on to suggest that there have been enough case studies to merit further research into the efficacy of clonidine for the treatment of autism. 124,125
For the treatment of schizophrenia via transdermal delivery, at least two drugs have been studied. Asenapine is a second-generation antipsychotic with 5HT-2A antagonist and 5HT-1A/1B partial agonist properties. In particular, transdermal asenapine was shown to have equal efficacy as compared to sublingual asenapine, but with all the advantages of transdermal delivery. Transdermal asenapine has already been approved by the FDA for use in schizophrenia treatment plans, but given its unique receptor profile, it is believed that transdermal asenapine could also have uses or value in the treatment of bipolar depression, anxiety, and aggression. 126,127 The second of the two drugs currently being transdermally delivered for schizophrenia is aripiprazole. Aripiprazole is a once-weekly, atypical antipsychotic. Multiple studies, discussed in a review paper, have validated the safety and efficacy of transdermal aripiprazole. 128
Perhaps the most obvious disadvantage of transdermal delivery lies within the strict requirements placed upon the drug of interest (DOI). Among other requirements, the DOI should have a fairly low molecular weight and a relatively high lipid solubility. Other disadvantages may include potentially slow time to peak plasma levels (i.e., unsuitable for emergency treatment), skin sensitivity and allergic reactions, patient compliance (i.e., willingness to abide by the patch exposure time requirements), and the fact that variations in skin could affect the quality of the patch’s adherence. Of course, the newer generations of transdermal delivery greatly combat many of those disadvantages. For instance, microneedle patches, third-generation transdermal patches, are designed to pierce the stratum corneum without penetrating deeply enough to stimulate pain receptors.129 In addition to maintaining all of the associated advantages of standard patches (e.g., self-administration, higher patient compliance, pain-free, tunable, etc.), microneedle patches open the door to an array of different or new drug classes. By puncturing and subsequently bypassing the skin’s most formidable physical barrier to drug delivery, microneedles make it possible to deliver larger and/or more hydrophilic drugs. Though dosage size is undoubtedly a limiting factor, there is a vast library of suitable drug candidates. Generally, the maximum drug dose for a microneedle patch sits around 1 mg, but that ceiling leaves enough room for a multitude of potent drugs ranging from vaccines (antigen, nanoparticle, and otherwise) to psychedelics. In addition to providing a novel and more patient-friendly delivery mechanism, microneedle patches have been shown to elicit a more robust immunological response when compared to conventional injections.130,131 Conventional vaccines are often injected into either the musculature or subcutaneous tissue, which are both highly capable of inducing a systemic immune response. However, microneedle patches can capitalize on the massive presence of antigen-presenting cells in the epidermis, a phenomenon that leads to microneedle patches often eliciting higher antibody production and cellular response.
Microneedle patches have only just recently been translated into the psychiatric space. In 2022, a group published a proof of concept for the usage of dissolving and solid microneedles for the in vivo delivery of iloperidone.132 Iloperidone, a WHO-approved schizophrenia drug, is both poorly soluble and bioavailable, two characteristics that make it a perfect candidate for microneedle delivery as opposed to oral. This research sought to compare both the antipsychotic activity and pharmacokinetics of iloperidone-loaded microneedle patches against a standard transdermal film. Two different microneedle patch types were developed and tested in preclinical studies. The dissolving microneedle patches, developed via micromolding, and the solid microneedle patches, developed via solvent casting, both demonstrated significant (p<0.001) improvements in the two measured responses (limb retraction times). The conclusion was that both the dissolving and solid microneedle designs improve efficacy for antipsychotic drug delivery while presenting no observed adverse effects.
Transdermal ketamine using either microporation or microneedles has also garnered significant interest. Outside of the perhaps more intuitive application in chronic pain management, studies have indicated that transdermal ketamine could also be used for the treatment of depression, bipolar disorders, and other psychiatric disorders. A research effort carried out in 2020 recognized the incoming wave of new antidepressants (two new classes of antidepressants were approved in 2019), so they decided to research the delivery mechanisms and pathways more thoroughly 119,133. They evaluated physical enhancement strategies such as iontophoresis, microneedle treatment, and ablative lasering. Their findings suggested that, for both ketamine and brexanolone, microporation of the skin using a laser was more effective (higher transdermal delivery) than microneedles. They were also able to achieve significantly higher percutaneous absorption with microemulsions of the DOIs. The paper goes on to argue that painless methods of delivery are even more important in patients that are already prone to anxiety.
3.2. Intranasal Delivery
The intranasal (IN) delivery of drugs intended to treat disorders of the brain creates an opportunity for both new and existing drugs to have faster onset, safer administration, and higher bioavailability. Though mostly discussed in a later section, it is important to note here that IN delivery is emerging as a remarkably useful tool for bypassing the blood-brain-barrier (BBB), hence the notably faster onset of IN-administered therapeutics and their ability to efficiently target the brain. More refined IN delivery techniques employing carriers, drug modifications, or both are opening the door for drugs that have not previously had the hydrophilic/phobic balance, solubility, or stability that is needed to achieve bioavailability via more traditional routes of administration.134 IN delivery is one of very few modern drug delivery approaches to make it into clinical psychiatry in a meaningful way, but there is still much work to be done in terms of translating recent advancements into the clinical setting.135
Among other implications, oxytocin has been shown to influence the reduction of addictive cravings as well as symptoms of both ASD and PTSD. In 2021, a group of researchers carried out a systematic review to determine if IN administration of oxytocin can reduce cravings in automated addictive behaviors.136 It was their belief that intranasal oxytocin warrants further investigation regarding its ability to dampen or eliminate acute stress-induced or withdrawal-related cravings (alcohol, cannabis, opioids, cocaine, nicotine, etc.). They also believe that IN oxytocin could play a role as ad hoc medication directly following drug-related cues. In 2021, a multilevel meta-analysis probed the usage of IN oxytocin for the treatment of ASD.137 This analysis looked at 28 studies with total N=726 ASD patients. Their findings suggested that IN oxytocin administration is an effective treatment for some core aspects of ASD: particularly within the domain of social functioning. Another study found that IN oxytocin given prior to psychotherapy sessions could be an effective additive in the treatment of PTSD.138 This conclusion arises from the theory that dampening the exaggerated fear response (via the reduction of amygdala hyperactivity) and enhancing top-down inhibitory control (via the promotion of prefrontal control over the amygdala) is a valuable strategy for the improvement of psychotherapy in PTSD patients, and it is believed that oxytocin acts on both of those processes.
The early success with IN oxytocin quickly encouraged similar research with IN ketamine, which has now been shown to improve depression and is thought likely to improve migraines, ASD, and other mood disorders. In 2021, a meta-analysis with n=858 was performed to assess the effect of IN ketamine on depression in adults, finding an antidepressant effect in unipolar depression (measured using the Montgomery-Asberg Depression Rating Scale) while maintaining mild or transient adverse effects.139 In another study, a large meta-analysis, suggests that patients receiving ketamine (intravenously) with esketamine (intranasally) experienced significantly fewer suicidal thoughts. That same meta-analysis proposes that more studies be done on IM/oral/sublingual ketamine to determine its ability to reduce suicidal ideation and the role that formulations/routes may or may not have.140 A study carried out in 2020 examined the antidepressant and anti-suicidal effect of IN esketamine and found that it had an ultra-rapid antidepressant effect for major depressive disorder patients, acting for at least 28 days. Though there is a relatively high level of confidence in the efficacy of IN esketamine, it is recommended that more research be done to analyze the dosing strategy and safety.141
IN dopamine has been studied for its effects on Parkinson’s, fear and anxiety, PTSD, and autism. In 2022, a study found that IN dopamine is a promising pharmacological tool in the psychotherapy of patients suffering from PTSD and suggests that it specifically alleviates fear associated with traumatic memories.142 In 2020, a study was done to see the therapeutic effect of IN dopamine in two distinct mouse models of autism. The results of this study suggested that IN dopamine effectively rectified the chosen behavioral phenotypes of both mouse models, further suggesting that this could be a promising therapy for diverse types of ASD.143
Intranasal neuropeptide Y (NPY) is also showing promising results in depression, PTSD, and other stress disorders. Neuropeptide Y is a peptide found in the brain of all mammals. Depressed models, rodent and human alike, have been shown to correlate with a reduced presence of neuropeptide Y mRNA and protein. This same correlation has also been observed in models of posttraumatic stress disorder and animals exposed to chronic stress and maternal separation. Recently, a randomized controlled trial observed major depressive disorder patients and the effects of IN neuropeptide Y. The results, using the Montgomery Asberg Depression Rating Scale (MADRS), overwhelmingly supported insufflated NPY as an antidepressant (mean difference of 4.778 using MADRS).144 Another study done in 2019 looked at the effects of IN NPY on the hyperarousal features seen in PTSD patients. They were able to convincingly demonstrate IN NPY’s ability to elicit long-lasting reversal of traumatic stress-triggered arousal.145 Shortly thereafter, it was realized that most of the studies on NPY had been done with male subjects even though females are just as, if not arguably more, prone to developing disorders such as PTSD, depression, anxiety disorders, and anorexia nervosa. They sought to review sex differences in the NPY system and understand the implications and impact on stress-related disorders. They were able to identify significant differences between sexes and put forth evidence that both may benefit from NPY treatment, but they ultimately hypothesized that, because of lower endogenous NPY expression on average, females would need a more concentrated formulation.146 These findings further support the need for highly personalized pharmacotherapies.
With all of the previously mentioned drugs, both new and old, it is important to consider implications and what their translation into newer delivery strategies means. Most fundamentally, these new strategies aim to circumvent the many obstacles presented by oral delivery: obstacles including lumen pH/enzymes and mucal clearance.147 Safely translating these drugs into either intranasal or transdermal delivery systems heightens bioavailability and can increase access to the brain, but it also creates opportunities for sustained release profiles, heightened targeting capabilities, and stimuli responsiveness.
3.3. Controlled-Release and Drug Targeting
It is important to note that controlled release technologies are intuitively combined with approaches for transdermal and IN delivery. For instance, the use of rate-controlling membranes is a common approach to tune the release rates of drugs from transdermal patches, while hydrogels can be used to tune release from microneedles.148,149 Controlled-release formulations have considerable utility for the treatment of mental disorders. Due to the long-term nature of most of the disorders, controlled-release formulations that could provide sustained drug delivery for months or even up to a year would be particularly useful. Moreover, patients with mental illness tend to struggle with drug compliance, and many of the medications have some potential for abuse and diversion.150 Many of the medications discussed herein also have narrow therapeutic windows, and poor pharmacokinetics of the parent drugs makes it very difficult to maintain systemic drug concentrations within the therapeutic window over time.151 These fluctuations in and out of the therapeutic window cause a major toll on patients, as they lead either to toxicities, lack of efficacy, or cycles between both. Therefore, many of the therapeutic settings we have discussed thus far would benefit immensely from the implementation of controlled drug release formulations.
One study looked at the IN delivery of lipid nanoparticle (LN) based fluoxetine hydrochloride.152 These LNs were nanostructured lipid carriers derived from oil-in-water emulsions where the oil was replaced by a mixture of solid lipids and liquid lipids and then stabilized by an aqueous emulsifier solution. By performing two behavioral tests, forced swimming and marble burying, they found that IN delivery of the optimized LN formulation performed similarly (no significant difference) to oral formulations. Given its ability to similarly reduce both depressive and anxiety-like behaviors, this sustained release formulation could prove highly valuable. It is thought that using LNs could provide a tailored and sustained drug release compatible with prolonged and more effective antidepressant effects. Simple and effective combinations of controlled release and/or drug targeting technologies with approaches to deliver drugs via the transdermal and IN routes are expected to provide synergistic benefits by more comprehensively overcoming the delivery barriers for psychiatric drugs.
Poly(lactide-co-glycolide) (PLGA)-based microspheres are biodegradable and biocompatible with the ability to sustain the delivery of small molecules and biologics.153 Naltrexone, an approved medication for treating or reducing symptoms of alcohol and opioid addiction, was loaded into PLGA microparticles with promising results.154 These PLGA microparticles were shown to release naltrexone for more than 40 days. The observed rate of drug release was not constant, but the variations in that rate were. Non-zero-order release was observed on days 0-10 where the drug was released via diffusion from the PLGA matrix but on days 10-35, the drug from the interior of the microsphere was released following zero-order kinetics. Another instance of PLGA in psychiatry includes the creation of PLGA microplates (square-based variants of microspheres that provide tunable mechanical properties and delayed release compared to PLGA microspheres) for the sustained delivery of risperidone (a commonly used antipsychotic) over several weeks.155 The taller of the two designed microplates was shown capable of inhibiting the cognitive deficit of mice for up to 3 months. The study suggested that risperidone microplates can be effective long-acting depots capable of releasing antipsychotic drugs in a controlled manner and ameliorating the dysbindin-induced deficits for at least 12 weeks.
In another work, hybrid copolymer polyvinyl alcohol (H-PVA) hydrogels intended for the controlled release of venlafaxine were produced.156 Though PVA is traditionally extremely soluble in water, it can be modified by crosslinking, grafting, blending, and copolymerization in order to greatly improve its resistance to aqueous degradation. Furthermore, three-dimensional hydrogel networks can be prepared by crosslinking hydroxyl groups of PVA. Venlafaxine is also traditionally extremely water-soluble and has to be administered two to three times daily because of its short circulation half-life. The results of this study suggested that venlafaxine-loaded H-PVA hydrogels could serve as valuable controlled release matrices for highly water-soluble antidepressants by providing pH-dependent “capture and release” of hydrophilic drugs with short half-lives. Importantly, it was found that the release rate of venlafaxine could be adjusted by varying the amounts of acrylic acid, 2-acrylamido-2-methyl propane sulfonic acid, and ethylene glycol dimethacrylate.
Another category of research that is extremely relevant to pharmacotherapy in mental health lies within the effort to better target drugs to the central nervous system (CNS). Cerebrospinal fluid, choroid plexus, and the BBB all pose anatomical or metabolic blockades between the peripheral circulation and the brain, but the BBB is perhaps the most burdensome in terms of drug delivery. The BBB is an anatomical protective barrier that minimizes the exchange of substances in brain cells.157 In its effort to reduce exchanges, the BBB uses tight junctions, adhesion molecules, and various enzymes as a metabolic barrier. Navigating the BBB and learning how to shuttle drugs safely across is crucial for the efficient treatment of a multitude of neurological disorders.
Targeted drug delivery to the brain can be broken down into three different classes of mediated transcytosis (absorptive-mediated, transporter-mediated, and receptor-mediated).158 Absorptive-mediated transcytosis (AMT) offers a pathway specifically for nanoparticles to cross the BBB. This arises as a result of the highly negatively charged luminal side of the BBB. AMT is promoted by electrostatic interactions between the negative moieties on the luminal surface of cerebral endothelial cells and cationic regions of the nanoparticle’s surface. Transporter-mediated transcytosis (TMT) provides yet another approach for the delivery of drugs to the brain. This relies on BBB-specific transporters and only works for the movement of drugs with low molecular weights from the bloodstream to the CNS. To utilize this phenomenon, nanoparticles or carriers can be conjugated with one of the 20 well-known BBB-specific transporters.158 The limitation of this technology lies in its tendency to prevent the uptake of needed nutrients. Finally, receptor-mediated transcytosis can be employed to overcome the BBB and deliver drugs to the brain tissue. This technique exploits receptor overexpression in the BBB and induces endocytosis through clathrin-coated pits or caveolae.
In 2021, a group published a paper that highlighted the formulation and clinical value of solid lipid nanoparticles (SLNs) as a new and biocompatible formulation strategy for shuttling CNS pharmaceuticals across the BBB.157 This approach offered highly controllable drug delivery, longer circulation time, targeting efficiency, and a higher efficacy in general all while increasing biocompatibility. The cited limitations with this technology are aligned with limitations often seen with SLNs: lower possible drug loading and decreased stability (gelation and drug expulsion). It is interesting to note that although these strategies have been shown to direct nanoparticles to the desired sites of action and can enable specific targeting to overexpressed transporters/receptors in the brain, there has yet to be any nanomedicine gain approval for the treatment of CNS disorders.158 In parallel with research being done on SLNs as a BBB bypass tool, preclinical research is being conducted on dendrimers, branched polymeric nanostructures, for their potential in the non-invasive crossing of the BBB. As a result of their significant branching, dendrimers offer many functional groups or sites that can be tailored to different drug delivery needs. Various groups have found that dendrimers can indeed target the CNS, and further research is being conducted on their ability to reduce the progression of various CNS diseases (Alzheimer’s, Parkinson’s, Prion disease, etc.). Current limitations to dendrimers include toxicity of traditional dendrimer chemistries such as polyamidoamine (PAMAM) and their scalability.159
Extracellular vesicles (EVs) encapsulate proteins, lipids, and nucleic acids, and can be found in virtually all biofluids.160 Given that EVs are naturally occurring and optimized for shuttling biomolecules, they make exceptional drug delivery candidates. Beyond generic drug delivery aptitude, EVs, in several different systems or compositions, have been found capable of targeted drug delivery to the CNS.161 When compared to synthetic nanoparticle-based drug delivery, EVs offer intrinsic properties of biocompatibility, lower immunogenicity, and higher stability in circulation. With them being able to efficiently cross the blood-brain barrier, EVs are increasingly being utilized as drug delivery vehicles for various CNS disorders. EVs derived from macrophages, microglia, and mesenchymal stem cells have been shown capable of specifically accumulating in inflamed brains.161 Though EVs represent an exciting new technology for CNS delivery, they possess limited drug loading capability compared to synthetic vehicles and can have considerable variability of performance between batches.162 Further, there are a range of difficulties surrounding the engineering, handling, and characterization of EVs that are likely to hamper scale up and clinical development of these technologies without further work.
4. Future Directions
4.1. Environmentally Responsive Delivery Systems
A major shortcoming of traditional controlled release technologies is their inability to respond to or change their behavior based on evolving pathophysiology. In other words, using traditional controlled release technology a drug will be constantly released whether it is needed or not. Therefore, environmentally-responsive drug delivery systems capable of responding to various physiological stimuli (pH, temperature, enzymes, inflammation, etc.) have been developed over the past couple decades.163 “Environmentally responsive”, as used in this discussion, is an umbrella term that covers two distinctly different categories: (1) systems that undergo a critical state change following administration, and (2) systems that respond to environmental cues or changes to initiate or terminate drug release. It is interesting to speculate on the various ways in which these systems could be incorporated into psychiatric drug delivery. For instance, materials that undergo a sol-gel transition at body temperature could be used as injectables or “sprayables” that can retain and release drug locally for long periods of time. Another example: materials that only degrade, and thus release drug, in response to reactive oxygen species (ROS) or other inflammatory molecules could be used to program drug release only in response to pathophysiological inflammatory cues. Below, we review a few early examples of studies that leveraged environmentally-responsive drug delivery systems to develop next-generation approaches for the treatment of mental illnesses.
Paliperidone (PLPD) is an approved drug for the treatment and/or management of schizophrenia. PLPD is an atypical antipsychotic with indications in both short and long-term treatment of schizophrenia. With the BBB inhibiting the delivery of therapeutics for so many CNS disorders, the IN route has become increasingly popular (as reviewed above). A group recently capitalized on the nasal passageway to the brain and implemented hydrogels with pH responsiveness for a sustained release effect.164 To prevent rapid mucociliary clearance, hydrogels were designed that undergo a sol-gel transition where the sprayable form was administered IN and, upon exposure to physiological pH, transitioned into a mucoadhesive gel for integration and long-term controlled release. The results presented in the study confirmed that the PLPD gel formulation achieved desirable drug permeation (in vitro and ex vivo) and safety.
Exciting results have also been achieved using thermoresponsive sol-gel systems.165 In 2021, a research group formulated self-assembled, thermosensitive in situ hydrogels (hydroxypropyl-β-cyclodextrin) containing both berberine and evodiamine.166 With berberine already having been shown as an effective IN medication, the main purpose of this study was to further reduce dosage requisites and symptoms while increasing sustainability by utilizing an intelligent drug delivery design that could prolong release. The in vitro results indicated that the prepared hydrogel maintained good thermosensitive properties under physiological temperature. The release profile most closely matched a first-order release mechanism, and the hydrogel showed a slow and controlled release of both the berberine and the evodiamine. They were able to show that the hydrogel formulation increased bioavailability by approximately 120-fold when compared to an intragastric administration. In 2021, a study was done on the formulation and efficacy of IN-administered, temperature-sensitive hydrogels of cannabidiol inclusion complex for the treatment of PTSD. 167 The chosen hydrogel was one made of poloxamer (nonionic triblock copolymers). The IN administration of these hydrogels was found to be more efficient and have more obvious brain-targeting effects than oral administration (for nasal administration, the maximum concentration of CBD in brain was found to be more than two times higher, and Cmax in the liver was halved), and it was reported that the hydrogels are both safe and effective as forms of controlled release. One research group formulated an intranasally delivered nortriptyline hydrochloride thermoreversible gelling system and characterized its effect on depression. They successfully created a highly stable hydrogel formulation that could be a valuable tool for improving patient compliance and treatment success.168 Another group produced environmentally responsive multilayer films based on block copolymer micelles and natural peptides. They did so to achieve the controlled release of favipiravir. These films were composed of polymer-coated silica nanocapsules (SNC) and poly(methacrylic acid) homopolymers. The films successfully exhibited swelling/deswelling behaviors under the trigger of a temperature stimulus. This work was the first to demonstrate temperature-responsive SNC-incorporated films with a well-defined internal structure and a sustained release profile.169
Interestingly, a hydrogel system was recently formulated that allows the controlled release of risperidone in response to electrical stimuli.170 This was an electroconductive, smart, polyacrylamide-polypyrrole hydrogel developed via electropolymerization of pyrrole inside the pores of a polyacrylamide hydrogel. The loaded hydrogel was characterized visually and then by swelling kinetics and network parameters. In vitro drug release studies and cytotoxic analyses on HepG2 and C6 cells supported both the kinetics and biocompatibility of the hydrogel. The hydrogel was shown to be able to control electrochemical phenomena for the liberation of risperidone molecules. The novelty of this research is its potential for translating into an implantable drug delivery device, or perhaps even a device for oral delivery, with the capability of controllable dosing via external signaling.
4.2. Pulsatile Delivery Systems
In the last few years, it has become increasingly clear that certain conditions require or greatly benefit from pulsatile delivery as opposed to constant or prolonged delivery. For example, psychedelics have recently emerged as a promising treatment for depression, anxiety, and mood disorders.100-106 To achieve successful dosing without triggering potentially dangerous or disorienting hallucinations, the psychedelic would ideally be microdosed on a pulsatile schedule. A comprehensive pulsatile drug delivery system should have high tunability (drug loading, release rates, profile combinations, etc.), either a self-sustained release mechanism or a highly reliable response to external stimuli, and exceptional biocompatibility. Beyond those baseline needs, it is expected that the system should either be easily passed, degraded, or retrieved. There are several ways by which pulsatile delivery can be categorized, but perhaps it is most useful to separate these methods by the stimuli that dictate their action. Pulsatile delivery is almost always dictated by one of three stimuli sources: time (constant, ambient conditions), environment (fluctuating or region-specific), and external factors (Figure 3).
Figure 3. Representative examples of pulsatile drug delivery systems.
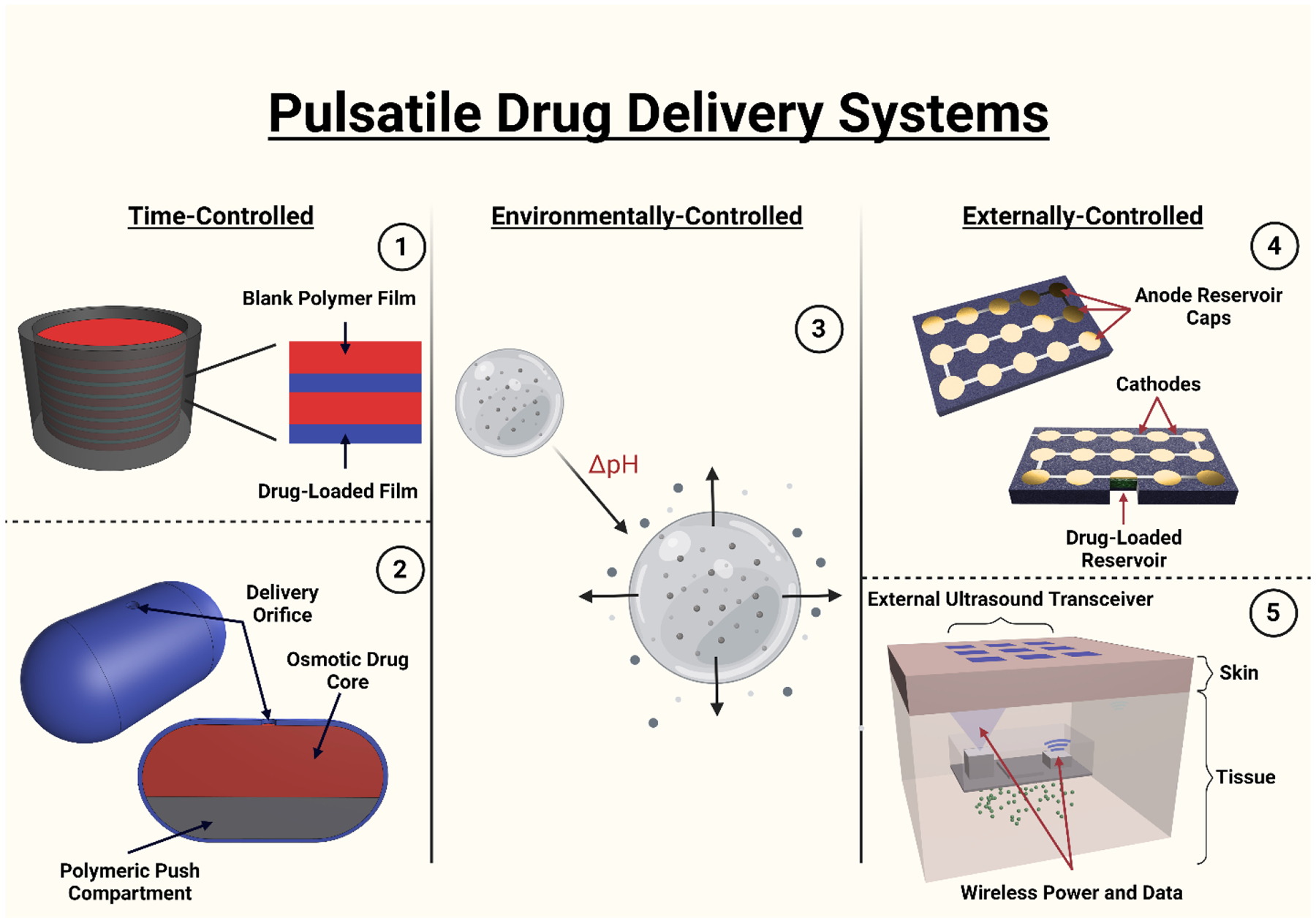
(1) Layered surface eroding implant, (2) Osmotic pump pill, (3) pH-responsive hydrogel (4) Anode/cathode thin film microchips (5) ultrasound-responsive delivery block.
In the 1990s, hydrogels began to be explored as a viable means of pulsatile drug delivery. Brazel and Peppas synthesized poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels for the pH- and temperature-sensitive drug delivery of antithrombotic agents.171 Both pH and temperature influenced the overall swelling behavior of the networks, which in turn dictated the release rates. Fluctuations in pH cause cyclic swelling and shrinking; that swelling and shrinking results in pulsatile drug delivery. Limitations in this design include drug size, drug denaturation, length of the release, and the temperamental nature of binding the active agent to the polymer network without hindering diffusion. The other clear limitation of this design is its dependency on regular and predictable environmental fluctuation to create pulsatile conditions.
Shortly thereafter, Tao and Desai published work on gastrointestinal patch systems with integrated bioadhesion, drug protection, and unidirectional release.172 The micropatches maximize the utilization of the intestine’s highly absorptive surface, and with a decreasing scale, this platform can go from being administered orally to being administered via injection. This technology can utilize the same phenomenon, swelling and shrinking as a result of pH changes, to effectively produce a pulsatile release profile.
Osmotic pumps or related systems have been another common approach to achieving timed release of compounds in vivo. These systems often relied on osmotic pressure and swelling to actuate the release of a medication from some capsule. Though most osmotic pumps are designed to achieve zero-order release, some groups have investigated ways to achieve a delayed release or pulsed release from osmotic systems as well. Niwa et al. prepared and published an ethyl cellulose capsule for the time-controlled release of drugs in the colon.173 Shortly thereafter, Jimoh et al. created biodegradable capsules out of poly(lactic acid) encapsulating citric acid/sodium bicarbonate with poly(lactide-co-glycolide) membranes.174 These osmotic-based capsules and the many other capsules designed using the same science have proven to be extremely useful. That having been said, the platform has a fair number of setbacks and limitations. Perhaps most notably, these capsules provide marginal protection against all of the obstacles associated with traditional oral drug delivery. Harsh conditions in the stomach, first-pass metabolism, and relatively rapid peristaltic shuttling make it difficult to achieve any truly long-term dosing schedules.
Controlled release technologies leading up to the advent of the microchip relied primarily on polymer science and chemical characteristics (biodegradability and responsiveness to environmental stimuli), but advances in microfabrication made it possible to create small, programmable devices. Santini et al. proposed and designed microchips with applications in a plethora of fields, including drug delivery.175 The drug delivery microchip consists of an array of reservoirs built into an electrolyte-impermeable substrate. The reservoirs are then sealed by a thin membrane that acts as an anode in electrochemical reactions. The device is then submerged in an electrolyte. When an electric potential is applied to an anode membrane, a soluble complex with the electrolyte ions is formed. The complex then dissolves, and the encapsulated drug or chemical is released.
Drug release technologies have relied heavily on bulk-eroding materials such as PLGA. More recently, however, there has been an increasing interest in surface-eroding materials for the controlled release of drugs. Surface-eroding materials are those materials where mass loss is linear and faster than the uptake of water into the bulk of the material. There are several advantages that surface-eroding materials provide. Their linear degradation at the interface or surface makes these materials extremely predictable in terms of degradation rates. These material properties open avenues to a multitude of geometries and designs. In the 1990s, it was discovered that blends of cellulose acetate phthalate (CAP) and Pluronic F-127 (PF-127) yielded a highly tunable, surface-eroding polymer.176 By stacking drug-loaded films with blank or unloaded films, a device can be turned into a pulsatile release system. Once the stacked device is coated on all sides but one, it creates a scenario in which erosion is unidirectional and highly predictable. The value of this device lies in its massive customizability. Films can be loaded with multiple drugs and with different release profiles. For example, one drug could undergo constant release while another drug is released once daily or weekly. In the early 2000s, this polymer blend (CAPP) was more proactively probed and explored as it relates to controlled drug delivery. This research started with an analysis of CAPP and how it could be tuned for the modulated release of bioactive proteins.177 That same group of people, or members of said group, have since then been exhaustively studying the polymer blend and further understanding its massive potential. They have now published research applying CAPP towards the intermittent release of simvastatin, different bioactive molecules, parathyroid hormone, and most recently, 5HT2A agonists.178-181 While these surface-eroding technologies have promise controlled, extended release and interval delivery as opposed to bolus release from bulk-eroding materials, there are some challenges associated with these materials. Surface-eroding materials change shape over time as degradation occurs, and are therefore not well-suited for environments where the device shape must remain constant. CAPP films specifically suffer from limited tunability since the ratio of CAP:P in the films and thickness of the films are the only levers by which you can tune release. Therefore, for very long-term extended release or for interval delivery over long periods of time, CAPP-based devices would become too thick to be practical for implantation.
The most recent ideation of CAPP films for interval dosing came to fruition as a result of recent clinical trials highlighting the effectiveness of 5HT2A agonists for the improvement of mood and psychotherapeutic growth in treatment-resistant depression patients. By alternating CAPP films loaded with 2,5-Dimethoxy-4-iodoamphetamine (DOI), a 5HT2A agonist, and unloaded or blank CAPP films, this research demonstrated the novel interval delivery of psychedelics from an implantable drug delivery system.181 In doing so, they opened the door to future studies on the delivery of therapeutic psychedelics. This technology or approach lends itself to psychiatric applications for multiple reasons. Given that the release of drugs from the device is entirely independent of outside factors, the procedure is extremely hands-off and would not require repeat visits. Given that the microdosing of psychedelics has so many implications in a group of patients with a heightened risk of compliance, it is important to have a drug delivery mechanism that is automated and virtually tamper-proof.
3D printing has changed the field in many ways, but most recently and most relevantly, it has been utilized for the fabrication of gastric anchors and floating drug delivery devices. Just in the last three years, there have been more than ten publications in this space.182 For situations where pulsatile delivery is needed and the conditions of the stomach do not denature or otherwise damage the DOI, anchors and floating devices could prove to be extremely valuable. A group was recently able to develop a floating, pulsatile drug delivery system via hot-melt extrusion and fused deposition modeling 3D printing.183 Hydroxypropyl cellulose and ethyl cellulose filaments were created and then those filaments were used for the FDM printing of shells around a theophylline (bronchodilator) tablet. In this model, shell thickness, wall thickness, and infill density can be changed to tune the release profile. All systems designed in this research effort exhibited good floating behavior with no lag time, and the time between pulses ranged from 30 minutes to six hours.
5. Conclusion
Mental illnesses such as anxiety disorders, autism spectrum disorder, post-traumatic stress disorder, schizophrenia, depression, and others place an immense burden on patients, their families, the economy, and society at large. Given that the United States spends an exorbitant amount of money on mental health and specifically depression, it is shocking that pharmaceutical strategies to treat mental illnesses are lagging so far behind drug development in other fields. However, the market for pharmaceutics in psychiatry is expected to grow rapidly over the coming decade. According to IBISWorld, the ADHD pharmaceutical market size is estimated to be $10.7 billion.184 Global Market Insights estimated the 2020 antidepressant market to be greater than $13.5 billion, with more than half of that being made off of SSRIs.185 Given that one of the primary obstacles in the advancement of therapeutics for mental illness relates to drug delivery issues, innovation in drug delivery technologies is critical. In this review, a number of mental health disorders and their current pharmacotherapies were discussed as well as several drug delivery approaches that have improved, in the laboratory, upon those existing therapies. Additionally, studies looking to improve drug bioavailability, provide controlled release of therapeutics, and/or enable drug targeting to the CNS were examined. That examination revealed a system of approaches, that once capitalized on and combined in a meaningful way, could transform psychiatry. In addition to discussing the recent research in psychiatric-specific drug delivery, next-generation drug delivery approaches having the potential to translate into psychiatry were brought forth for analysis. Discussed approaches included environmentally-controlled release designs as well as pulsatile delivery systems controlled via hydrolysis, environmental stimuli, and/or external factors. Based on the evidence as seen in this literature review, the field of drug delivery in mental health appears to be at the beginning of a much-needed awakening.
Acknowledgments
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number P30 GM122733-03A1. This work was also supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) at the NIH under the award number R21EB031454-01A1. Figures in the article were created with BioRender.com.
References
- 1.Kenwood MM, Kalin NH & Barbas H The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 47, 260–275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ströhle A, Gensichen J & Domschke K The Diagnosis and Treatment of Anxiety Disorders. Dtsch Arztebl Int (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocq M-A The history of generalized anxiety disorder as a diagnostic category. Dialogues Clin Neurosci 19, 107–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strawn JR, Geracioti L, Rajdev N, Clemenza K & Levine A Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother 19, 1057–1070 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinaugh DJ et al. Towards a precision psychiatry approach to anxiety disorders with ecological momentary assessment: the example of panic disorder. Gen Psychiatr 33, e100161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune T, Nordahl HM & Beidel DC Social anxiety disorder in adolescents: Prevalence and subtypes in the Young-HUNT3 study. J Anxiety Disord 87, 102546 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Eaton WW, Bienvenu OJ & Miloyan B Specific phobias. Lancet Psychiatry 5, 678–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein DJ, Scott KM, de Jonge P & Kessler RC Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci 19, 127–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibaut F. The role of sex and gender in neuropsychiatric disorders. Dialogues Clin Neurosci 18, 351–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandelow B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. in 347–365 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Thibaut F. Anxiety disorders: a review of current literature. Dialogues Clin Neurosci 19, 87–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoeff B. Fundamental challenges for autism research: the science–practice gap, demarcating autism and the unsuccessful search for the neurobiological basis of autism. Med Health Care Philos 18, 443–447 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Thurm A, Farmer C, Salzman E, Lord C & Bishop S State of the Field: Differentiating Intellectual Disability From Autism Spectrum Disorder. Front Psychiatry 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caçola P, Miller HL & Williamson PO Behavioral comparisons in Autism Spectrum Disorder and Developmental Coordination Disorder: A systematic literature review. Res Autism Spectr Disord 38, 6–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brukner-Wertman Y, Laor N & Golan O Social (Pragmatic) Communication Disorder and Its Relation to the Autism Spectrum: Dilemmas Arising From the DSM-5 Classification. J Autism Dev Disord 46, 2821–2829 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Rau S. et al. Identifying comorbid ADHD in autism: Attending to the inattentive presentation. Res Autism Spectr Disord 69, 101468 (2020). [Google Scholar]
- 17.Martino D, Ganos C & Pringsheim TM Tourette Syndrome and Chronic Tic Disorders: The Clinical Spectrum Beyond Tics. in 1461–1490 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Fernell E, Eriksson & Gillberg C Early diagnosis of autism and impact on prognosis: a narrative review. Clin Epidemiol 33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing L. The definition and prevalence of autism: A review. Eur Child Adolesc Psychiatry 2, 61–74 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Rice CE et al. Defining in Detail and Evaluating Reliability of DSM-5 Criteria for Autism Spectrum Disorder (ASD) Among Children. J Autism Dev Disord 52, 5308–5320 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salari N. et al. The global prevalence of autism spectrum disorder: a comprehensive systematic review and meta-analysis. Ital J Pediatr 48, 112 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillberg IC & Gillberg C Asperger Syndrome?Some Epidemiological Considerations: A Research Note. Journal of Child Psychology and Psychiatry 30, 631–638 (1989). [DOI] [PubMed] [Google Scholar]
- 23.Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry 66 Suppl 1, 3–8 (2005). [PubMed] [Google Scholar]
- 24.Kopp S, Kelly KB & Gillberg C Girls With Social and/or Attention Deficits: A Descriptive Study of 100 Clinic Attenders. J Atten Disord 14, 167–181 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Yehuda R. Post-Traumatic Stress Disorder. New England Journal of Medicine 346, 108–114 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Pitman RK et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13, 769–787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalev A, Liberzon I & Marmar C Post-Traumatic Stress Disorder. New England Journal of Medicine 376, 2459–2469 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Bryant RA Post-traumatic stress disorder: a state-of-the-art review of evidence and challenges. World Psychiatry 18, 259–269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girgenti MJ et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci 24, 24–33 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Kaseda ET & Levine AJ Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol 34, 1498–1514 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Fountoulakis KN et al. The CINP Guidelines on the Definition and Evidence-Based Interventions for Treatment-Resistant Bipolar Disorder. International Journal of Neuropsychopharmacology 23, 230–256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordovez FJA & McMahon FJ The genetics of bipolar disorder. Mol Psychiatry 25, 544–559 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Rowland TA & Marwaha S Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol 8, 251–269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiskal HS Classification, Diagnosis and Boundaries of Bipolar Disorders. in Bipolar Disorder 1–95 (John Wiley & Sons, Ltd, 2002). [Google Scholar]
- 35.Carvalho AF, Firth J & Vieta E Bipolar Disorder. New England Journal of Medicine 383, 58–66 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Morris G. et al. Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev 107, 862–882 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Yee CS, Hawken ER, Baldessarini RJ & Vázquez GH Maintenance Pharmacological Treatment of Juvenile Bipolar Disorder: Review and Meta-Analyses. International Journal of Neuropsychopharmacology 22, 531–540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daveney J, Panagioti M, Waheed W & Esmail A Unrecognized bipolar disorder in patients with depression managed in primary care: A systematic review and meta-analysis. Gen Hosp Psychiatry 58, 71–76 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Insel TR Rethinking schizophrenia. Nature 468, 187–193 (2010). [DOI] [PubMed] [Google Scholar]
- 40.McCutcheon RA, Reis Marques T & Howes OD Schizophrenia—An Overview. JAMA Psychiatry 77, 201 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Fusar-Poli P & Politi P Paul Eugen Bleuler and the Birth of Schizophrenia (1908). American Journal of Psychiatry 165, 1407–1407 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Provencher HL & Mueser KT Positive and negative symptom behaviors and caregiver burden in the relatives of persons with schizophrenia. Schizophr Res 26, 71–80 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Saha S, Chant D, Welham J & McGrath J A Systematic Review of the Prevalence of Schizophrenia. PLoS Med 2, e141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cloutier M. et al. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 77, 764–771 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Hjorthøj C, Stürup AE, McGrath JJ & Nordentoft M Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 4, 295–301 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Sher L & Kahn RS Suicide in Schizophrenia: An Educational Overview. Medicina (B Aires) 55, 361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vohra J. Sudden Cardiac Death in Schizophrenia: A Review. Heart Lung Circ 29, 1427–1432 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Otte C. et al. Major depressive disorder. Nat Rev Dis Primers 2, 16065 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Seedat S. et al. Cross-National Associations Between Gender and Mental Disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry 66, 785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vos T. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 386, 743–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattina GF, Van Lieshout RJ & Steiner M Inflammation, depression and cardiovascular disease in women: the role of the immune system across critical reproductive events. Ther Adv Cardiovasc Dis 13, 175394471985195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiweck C. et al. Depression and suicidality: A link to premature T helper cell aging and increased Th17 cells. Brain Behav Immun 87, 603–609 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Flint J & Kendler KS The Genetics of Major Depression. Neuron 81, 484–503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner MJ, Thomas HJ & Erskine HE The association between five forms of child maltreatment and depressive and anxiety disorders: A systematic review and meta-analysis. Child Abuse Negl 96, (2019). [DOI] [PubMed] [Google Scholar]
- 55.Lima-Ojeda JM, Rupprecht R & Baghai TC Neurobiology of depression: A neurodevelopmental approach. The World Journal of Biological Psychiatry 19, 349–359 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Greenberg PE et al. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 39, 653–665 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinyor M, Schaffer A & Levitt A The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Trial: A Review. The Canadian Journal of Psychiatry 55, 126–135 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Conway CR, George MS & Sackeim HA Toward an Evidence-Based, Operational Definition of Treatment-Resistant Depression. JAMA Psychiatry 74, 9 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53, 649–659 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Amital D, Fostick L, Silberman A, Beckman M & Spivak B Serious life events among resistant and non-resistant MDD patients. J Affect Disord 110, 260–264 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Amos TB et al. Direct and Indirect Cost Burden and Change of Employment Status in Treatment-Resistant Depression. J Clin Psychiatry 79, 24–32 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Cusin C & Dougherty DD Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord 2, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gnanavel S, Sharma P, Kaushal P & Hussain S Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J Clin Cases 7, 2420–2426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayashi W et al. ASD symptoms in adults with ADHD: a preliminary study using the ADOS-2. Eur Arch Psychiatry Clin Neurosci 272, 217–232 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Faraone SV & Larsson H Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 24, 562–575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koyuncu A, Ayan T, Ince Guliyev E, Erbilgin S & Deveci E ADHD and Anxiety Disorder Comorbidity in Children and Adults: Diagnostic and Therapeutic Challenges. Curr Psychiatry Rep 24, 129–140 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Schiweck C. et al. Comorbidity of ADHD and adult bipolar disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev 124, 100–123 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Sonuga-Barke EJS et al. Annual Research Review: Perspectives on progress in ADHD science – from characterization to cause. Journal of Child Psychology and Psychiatry (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ronald A, de Bode N & Polderman TJC Systematic Review: How the Attention-Deficit/Hyperactivity Disorder Polygenic Risk Score Adds to Our Understanding of ADHD and Associated Traits. J Am Acad Child Adolesc Psychiatry 60, 1234–1277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solberg BS et al. Gender differences in psychiatric comorbidity: a population-based study of 40 000 adults with attention deficit hyperactivity disorder. Acta Psychiatr Scand 137, 176–186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faheem M. et al. Gender-based differences in prevalence and effects of ADHD in adults: A systematic review. Asian J Psychiatr 75, 103205 (2022). [DOI] [PubMed] [Google Scholar]
- 72.Stavropoulos V. et al. Associations between attention deficit hyperactivity and internet gaming disorder symptoms: Is there consistency across types of symptoms, gender and countries? Addictive Behaviors Reports 9, 100158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandelow B. et al. Efficacy of treatments for anxiety disorders. Int Clin Psychopharmacol 30, 183–192 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Garakani A. et al. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front Psychiatry 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou MS et al. Meta-analysis: Pharmacologic Treatment of Restricted and Repetitive Behaviors in Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry 60, 35–45 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Shiner B. et al. Mining Clinical Data for Novel Posttraumatic Stress Disorder Medications. Biol Psychiatry 91, 647–657 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kato T. Current understanding of bipolar disorder: Toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci 73, 526–540 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Morinigo J-D, Leucht S & Arango C Pharmacological Treatment of Early-Onset Schizophrenia: A Critical Review, Evidence-Based Clinical Guidance and Unmet Needs. Pharmacopsychiatry 55, 233–245 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karrouri R, Hammani Z, Benjelloun R & Otheman Y Major depressive disorder: Validated treatments and future challenges. World J Clin Cases 9, 9350–9367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown KA, Samuel S & Patel DR Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: a review for practitioners. Transl Pediatr 7, 36–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parsaik AK et al. Mortality associated with anxiolytic and hypnotic drugs—A systematic review and meta-analysis. Australian & New Zealand Journal of Psychiatry 50, 520–533 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Turner M. The role of drugs in the treatment of autism. Aust Prescr 43, 185–190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCormack G, Dillon AC, Healy O, Walsh C & Lydon S Primary Care Physicians’ Knowledge of Autism and Evidence-Based Interventions for Autism: A Systematic Review. Rev J Autism Dev Disord 7, 226–241 (2020). [Google Scholar]
- 84.Henneberry E, Lamy M, Dominick KC & Erickson CA Decades of Progress in the Psychopharmacology of Autism Spectrum Disorder. J Autism Dev Disord 51, 4370–4394 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Tural Hesapcioglu S, Ceylan MF, Kasak M & Sen CP Olanzapine, risperidone, and aripiprazole use in children and adolescents with Autism Spectrum Disorders. Res Autism Spectr Disord 72, 101520 (2020). [Google Scholar]
- 86.Yehuda R. et al. Post-traumatic stress disorder. Nat Rev Dis Primers 1, 15057 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Hoskins M. et al. Pharmacotherapy for post-traumatic stress disorder: Systematic review and meta-analysis. British Journal of Psychiatry 206, 93–100 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Astill Wright L. et al. Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Transl Psychiatry 9, 334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yildiz A, Nikodem M, Vieta E, Correll CU & Baldessarini RJ A network meta-analysis on comparative efficacy and all-cause discontinuation of antimanic treatments in acute bipolar mania. Psychol Med 45, 299–317 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Grande I & Vieta E Pharmacotherapy of Acute Mania: Monotherapy or Combination Therapy with Mood Stabilizers and Antipsychotics? CNS Drugs 29, 221–227 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Rosenblat JD et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord 18, 89–101 (2016). [DOI] [PubMed] [Google Scholar]
- 92.McGirr A, Vöhringer PA, Ghaemi SN, Lam RW & Yatham LN Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry 3, 1138–1146 (2016). [DOI] [PubMed] [Google Scholar]
- 93.El-Tokhy FS, Abdel-Mottaleb MMA, El-Ghany EA & Geneidi AS Transdermal delivery of second-generation antipsychotics for management of schizophrenia; disease overview, conventional and nanobased drug delivery systems. J Drug Deliv Sci Technol 61, 102104 (2021). [Google Scholar]
- 94.Zorkina Y. et al. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules 25, 5294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kato M. et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry 26, 118–133 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thom R, Silbersweig DA & Boland RJ Major Depressive Disorder in Medical Illness: A Review of Assessment, Prevalence, and Treatment Options. Psychosom Med 81, 246–255 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Altaf R, Gonzalez I, Rubino K & Nemec EC Folate as adjunct therapy to SSRI/SNRI for major depressive disorder: Systematic review & meta-analysis. Complement Ther Med 61, 102770 (2021). [DOI] [PubMed] [Google Scholar]
- 98.Ichikawa N. et al. Primary functional brain connections associated with melancholic major depressive disorder and modulation by antidepressants. Sci Rep 10, 3542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhdanava M. et al. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry 82, (2021). [DOI] [PubMed] [Google Scholar]
- 100.Carhart-Harris RL et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3, 619–627 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Carhart-Harris RL et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl) 235, 399–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carhart-Harris R & Nutt D Serotonin and brain function: a tale of two receptors. Journal of Psychopharmacology 31, 1091–1120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carhart-Harris RL & Goodwin GM The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 42, 2105–2113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK & Muthukumaraswamy SD Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7, 46421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carhart-Harris RL et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep 7, 13187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daws RE et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med 28, 844–851 (2022). [DOI] [PubMed] [Google Scholar]
- 107.Hibicke M, Landry AN, Kramer HM, Talman ZK & Nichols CD Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci 11, 864–871 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Cortese S. et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 5, 727–738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sibley MH et al. Variable Patterns of Remission From ADHD in the Multimodal Treatment Study of ADHD. American Journal of Psychiatry 179, 142–151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lopez PL et al. Cognitive-behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2018, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elliott J. et al. Pharmacologic treatment of attention deficit hyperactivity disorder in adults: A systematic review and network meta-analysis. PLoS One 15, e0240584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigues R. et al. Practitioner Review: Pharmacological treatment of attention-deficit/hyperactivity disorder symptoms in children and youth with autism spectrum disorder: a systematic review and meta-analysis. Journal of Child Psychology and Psychiatry 62, 680–700 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Anbarasan D, Safyer G & Adler LA Updates in Pharmacologic Strategies in Adult Attention-Deficit/Hyperactivity Disorder. Child Adolesc Psychiatr Clin N Am 31, 553–568 (2022). [DOI] [PubMed] [Google Scholar]
- 114.Ivanov I, Bjork JM, Blair J & Newcorn JH Sensitization-based risk for substance abuse in vulnerable individuals with ADHD: Review and re-examination of evidence. Neurosci Biobehav Rev 135, 104575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bahn GH & Seo K Combined Medication with Stimulants and Non-stimulants for Attention-deficit/hyperactivity Disorder. Clinical Psychopharmacology and Neuroscience 19, 705–711 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siffel C. et al. Suicidal ideation and attempts in the United States of America among stimulant-treated, non-stimulant-treated, and untreated patients with a diagnosis of attention-deficit/hyperactivity disorder. J Affect Disord 266, 109–119 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Prausnitz MR & Langer R Transdermal drug delivery. Nature Biotechnology vol. 26 1261–1268 Preprint at 10.1038/nbt.1504 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bodkin JA & Amsterdam JD Transdermal selegiline in major depression: A double-blind, placebo-controlled, parallel-group study in outpatients. American Journal of Psychiatry 159, 1869–1875 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Lu BY et al. Rapid and sustained improvement in treatment-refractory depression through use of acute intravenous ketamine and concurrent transdermal selegiline: A case series. J Affect Disord 262, 40–42 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Zarzar M. et al. Transdermal selegiline Transdermal Selegiline: The New Generation of Monoamine Oxidase Inhibitors. [Google Scholar]
- 121.25981_treatment-social-phobia-antidepressants. [Google Scholar]
- 122.Joseph McClernon F, Berry Hiott F, Westman EC, Rose JE & Levin ED Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 123.van Schalkwyk GI et al. Reduction of Aggressive Episodes After Repeated Transdermal Nicotine Administration in a Hospitalized Adolescent with Autism Spectrum Disorder. J Autism Dev Disord 45, 3061–3066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye L & Lippmann S Reduction of anxiety after treatment with transdermal clonidine. American Journal of Health-System Pharmacy 75, 742–744 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Banas K & Sawchuk B Clonidine as a Treatment of Behavioural Disturbances in Autism Spectrum Disorder: A Systematic Literature Review. Journal of the Canadian Academy of Child and Adolescent Psychiatry 29, 110 (2020). [PMC free article] [PubMed] [Google Scholar]
- 126.Carrithers B & El-Mallakh RS Transdermal Asenapine in Schizophrenia: A Systematic Review. (2020) doi: 10.2147/PPA.S235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou M. et al. Asenapine Transdermal Patch for the Management of Schizophrenia. Psychopharmacol Bull 50, 60 (2020). [PMC free article] [PubMed] [Google Scholar]
- 128.Citrome L, Courtney, ;, Zeni M & Correll CU Expert Commentary Patches: Established and Emerging Transdermal Treatments in Psychiatry. (2019) doi: 10.4088/JCP.18nr12554. [DOI] [PubMed] [Google Scholar]
- 129.Opinion: An Alternative to Injection. https://www.the-scientist.com (1986).
- 130.Quan F-S et al. Intradermal Vaccination with Influenza Virus-Like Particles by Using Microneedles Induces Protection Superior to That with Intramuscular Immunization. J Virol 84, 7760–7769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vrdoljak A. et al. Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods. Journal of Controlled Release 225, 192–204 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Bhadale RS, Vaishali, · & Londhe Y A comparison of dissolving microneedles and transdermal film with solid microneedles for iloperidone in vivo: a proof of concept. Naunyn Schmiedebergs Arch Pharmacol doi: 10.1007/s00210-022-02309-0. [DOI] [PubMed] [Google Scholar]
- 133.Bhattaccharjee SA, Murnane KS & Banga AK Transdermal delivery of breakthrough therapeutics for the management of treatment-resistant and post-partum depression. Int J Pharm 591, 120007 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Lofts A. et al. Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs. CNS Drugs 2022 36:7 36, 739–770 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vitorino C, Silva S, Bicker J, Falcão A & Fortuna A Antidepressants and nose-to-brain delivery: drivers, restraints, opportunities and challenges. Drug Discov Today 24, 1911–1923 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Houghton B, Kouimtsidis C, Duka T, Paloyelis Y & Bailey A Can intranasal oxytocin reduce craving in automated addictive behaviours? A systematic review. Br J Pharmacol 178, 4316–4334 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Huang Y, Huang X, Ebstein RP & Yu R Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis. Neurosci Biobehav Rev 122, 18–27 (2021). [DOI] [PubMed] [Google Scholar]
- 138.Koch SBJ et al. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: Salience processing and fear inhibition processes. Psychoneuroendocrinology 40, 242–256 (2014). [DOI] [PubMed] [Google Scholar]
- 139.An D, Wei C, Wang J & Wu A Intranasal Ketamine for Depression in Adults: A Systematic Review and Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. Front Psychol 12, 1520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiong J. et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: A systematic review and meta-analysis. J Psychiatr Res 134, 57–68 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Zheng W. et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 265, 63–70 (2020). [DOI] [PubMed] [Google Scholar]
- 142.de Almeida Silva M. et al. The activation of D2-like receptors by intranasal dopamine facilitates the extinction of contextual fear and prevents conditioned fear-induced antinociception. Behavioural Brain Research 417, 113611 (2022). [DOI] [PubMed] [Google Scholar]
- 143.Chao OY et al. Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol Brain 13, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mathé AA, Michaneck M, Berg E, Charney DS & Murrough JW A Randomized Controlled Trial of Intranasal Neuropeptide Y in Patients With Major Depressive Disorder. International Journal of Neuropsychopharmacology 23, 783–790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nwokafor C, Serova LI & Sabban EL Preclinical findings on the potential of intranasal neuropeptide Y for treating hyperarousal features of PTSD. Ann N Y Acad Sci 1455, 149–159 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Nahvi RJ & Sabban EL Sex Differences in the Neuropeptide Y System and Implications for Stress Related Disorders. Biomolecules 2020, Vol. 10, Page 1248 10, 1248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Homayun B, Lin X & Choi H-J Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bolzinger MA, Briançon S, Pelletier J & Chevalier Y Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci 17, 156–165 (2012). [Google Scholar]
- 149.Dreiss CA Hydrogel design strategies for drug delivery. Curr Opin Colloid Interface Sci 48, 1–17 (2020). [Google Scholar]
- 150.Velligan DI et al. The Expert Consensus Guideline Series: Adherence Problems in Patients with Serious and Persistent Mental Illness Editors for the Guidelines. (2009). [PubMed] [Google Scholar]
- 151.de Leon J. The future (or lack of future) of personalized prescription in psychiatry. Pharmacol Res 59, 81–89 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Vitorino C. et al. QbD-driven development of intranasal lipid nanoparticles for depression treatment. European Journal of Pharmaceutics and Biopharmaceutics 153, 106–120 (2020). [DOI] [PubMed] [Google Scholar]
- 153.Andhariya JV et al. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. Journal of Controlled Release 255, 27–35 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Sharifi F, Otte A, Yoon G & Park K Continuous in-line homogenization process for scale-up production of naltrexone-loaded PLGA microparticles. Journal of Controlled Release 325, 347–358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bellotti E. et al. Long-lasting rescue of schizophrenia-relevant cognitive impairments via risperidone-loaded microPlates. Drug Deliv Transl Res 12, 1829 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ali L, Ahmad M, Usman M & Yousuf M Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polymer Bulletin 71, 31–46 (2014). [Google Scholar]
- 157.Kumar Satapathy M. et al. pharmaceutics Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. (2021) doi: 10.3390/pharmaceutics13081183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinheiro RGR, Coutinho AJ, Pinheiro M & Neves AR Molecular Sciences Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? (2021) doi: 10.3390/ijms222111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Palan F & Chatterjee B Dendrimers in the context of targeting central nervous system disorders. J Drug Deliv Sci Technol 73, 103474 (2022). [Google Scholar]
- 160.Bari SMI, Hossain FB & Nestorova GG Advances in Biosensors Technology for Detection and Characterization of Extracellular Vesicles. Sensors 2021, Vol. 21, Page 7645 21, 7645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou L. et al. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals 2022, Vol. 15, Page 358 15, 358 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vader P, Mol EA, Pasterkamp G & Schiffelers RM Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 106, 148–156 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Adepu S & Ramakrishna S Controlled drug delivery systems: Current status and future directions. Molecules vol. 26 Preprint at 10.3390/molecules26195905 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sherje AP & Londhe V Development and Evaluation of pH-Responsive Cyclodextrin-Based in situ Gel of Paliperidone for Intranasal Delivery. doi: 10.1208/s12249-017-0844-8. [DOI] [PubMed] [Google Scholar]
- 165.Agrawal M. et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. Journal of Controlled Release vol. 327 235–265 Preprint at 10.1016/j.jconrel.2020.07.044 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Xu D, Qiu C, Wang Y, Qiao T & Cui YL Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int J Pharm 603, 120667 (2021). [DOI] [PubMed] [Google Scholar]
- 167.Pang L. et al. Intranasal temperature-sensitive hydrogels of cannabidiol inclusion complex for the treatment of post-traumatic stress disorder. Acta Pharm Sin B 11, 2031–2047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pathan IB & More B Formulation And Characterization Of Intra Nasal Delivery Of Nortriptyline Hydrochloride Thermoreversible Gelling System In Treatment Of Depression. Acta Pharmaceutica Sciencia 55, (2017). [Google Scholar]
- 169.Xu L. et al. Environmentally Responsive Multilayer Films Based on Block Copolymer Micelles and Natural Peptides for Controlled Release of Favipiravir. [Google Scholar]
- 170.Saha S, Sarkar P, Sarkar M & Giri B Electroconductive smart polyacrylamide–polypyrrole (PAC–PPY) hydrogel: a device for controlled release of risperidone. RSC Adv 5, 27665–27673 (2015). [Google Scholar]
- 171.Brazel CS & Peppas NA Pulsatile local delivery of thrombolytic and antithrombotic agents using poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. Journal of Controlled Release 39, 57–64 (1996). [Google Scholar]
- 172.Tao SL & Desai TA Gastrointestinal patch systems for oral drug delivery. Drug Discov Today 10, 909–915 (2005). [DOI] [PubMed] [Google Scholar]
- 173.Niwa K, Takaya T, Morimoto T & Takada K Preparation and Evaluation of a Time-Controlled Release Capsule Made of Ethylcellulose for Colon Delivery of Drugs. 10.3109/10611869509059209 3, 83–89 (2008). [DOI] [PubMed] [Google Scholar]
- 174.Ganiyu Jimoh A, Wise DL, Gresser JD & Trantolo DJ Pulsed FSH release from an implantable capsule system. Journal of Controlled Release 34, 87–95 (1995). [Google Scholar]
- 175.Santini JT, Richards AC, Scheidt R, Cima MJ & Langer R Microchips as controlled drug-delivery devices. Angewandte Chemie - International Edition vol. 39 2396–2407 Preprint at (2000). [DOI] [PubMed] [Google Scholar]
- 176.Xu X & Lee PI Programmable Drug Delivery from an Erodible Association Polymer System. Pharmaceutical Research: An Official Journal of the American Association of Pharmaceutical Scientists 10, 1144–1152 (1993). [DOI] [PubMed] [Google Scholar]
- 177.Raiche A & Puleo D Association polymers for modulated release of bioactive proteins. (2003) doi: 10.1109/MEMB.2003.1256270. [DOI] [PubMed] [Google Scholar]
- 178.Jeon JH, Thomas MV & Puleo DA Bioerodible devices for intermittent release of simvastatin acid. Int J Pharm 340, 6–12 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jeon JH & Puleo DA Alternating Release of Different Bioactive Molecules from a Complexation Polymer System. Biomaterials 29, 3591 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jeon JH & Puleo DA Formulations for intermittent release of parathyroid hormone (1-34) and local enhancement of osteoblast activities. Pharm Dev Technol 13, 505–512 (2008). [DOI] [PubMed] [Google Scholar]
- 181.Hossain M. et al. Interval delivery of 5HT2A agonists using multilayered polymer films. J Biomed Mater Res A (2023) doi: 10.1002/JBM.A.37497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.View of Recent advances in 3D printing for floating drug delivery platforms. https://li01.tci-thaijo.org/index.php/sehs/article/view/252666/174599. [Google Scholar]
- 183.Reddy Dumpa N, Bandari S & Repka MA pharmaceutics Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. doi: 10.3390/pharmaceutics12010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.ADHD Medication Manufacturing in the US - Market Size ∣ IBISWorld. https://www.ibisworld.com/industry-statistics/market-size/adhd-medication-manufacturing-united-states/. [Google Scholar]
- 185.Antidepressant Drugs Market Size & Share, Forecast Report 2021-2027. https://www.gminsights.com/industry-analysis/antidepressant-drugs-market.


