Abstract
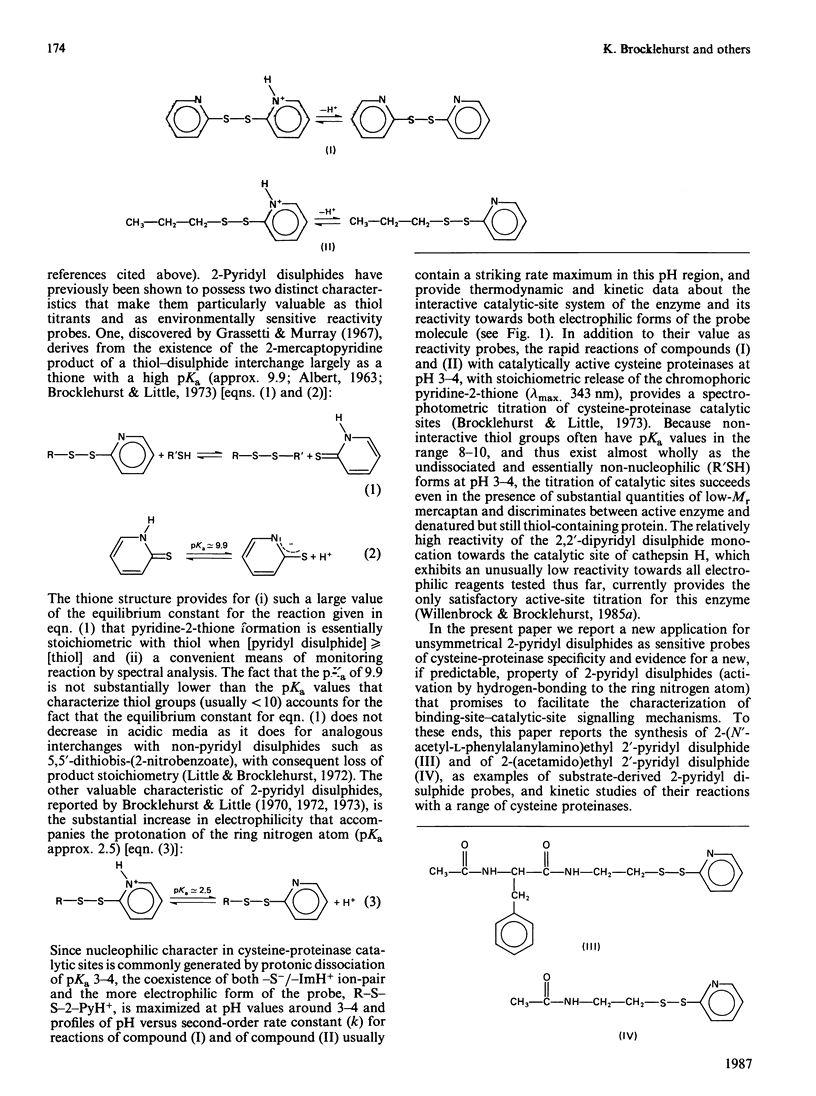
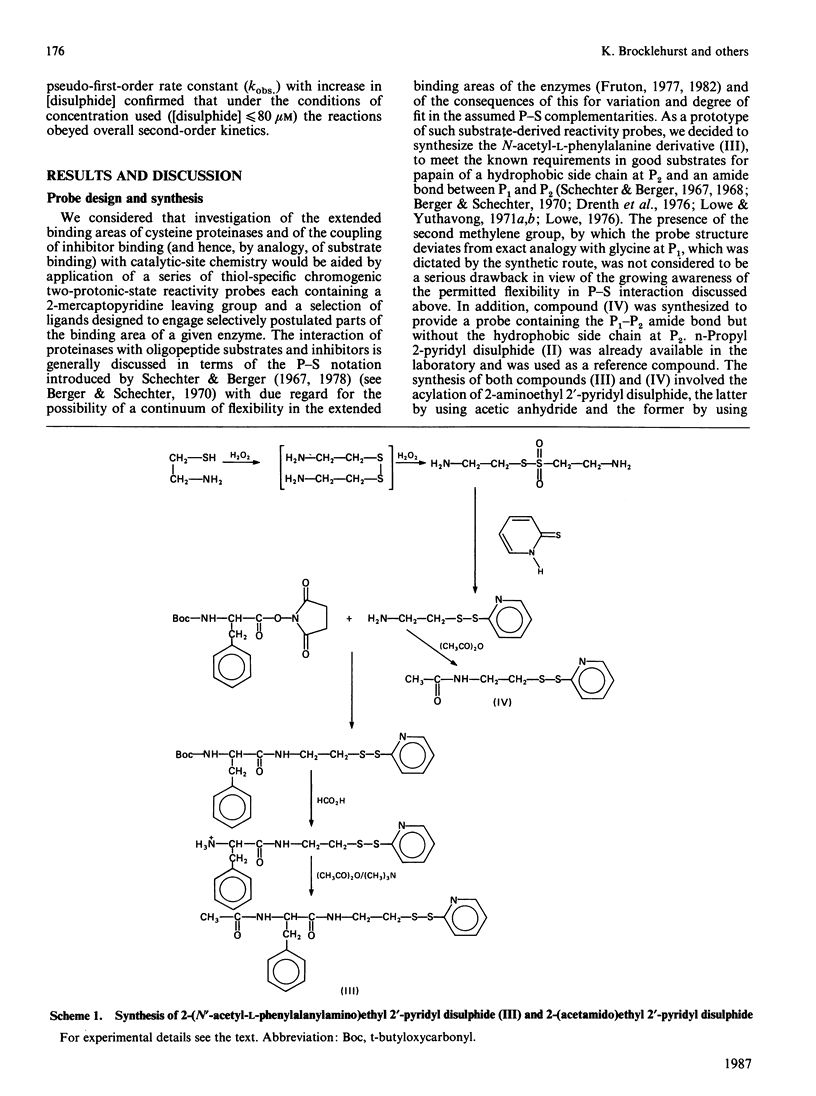
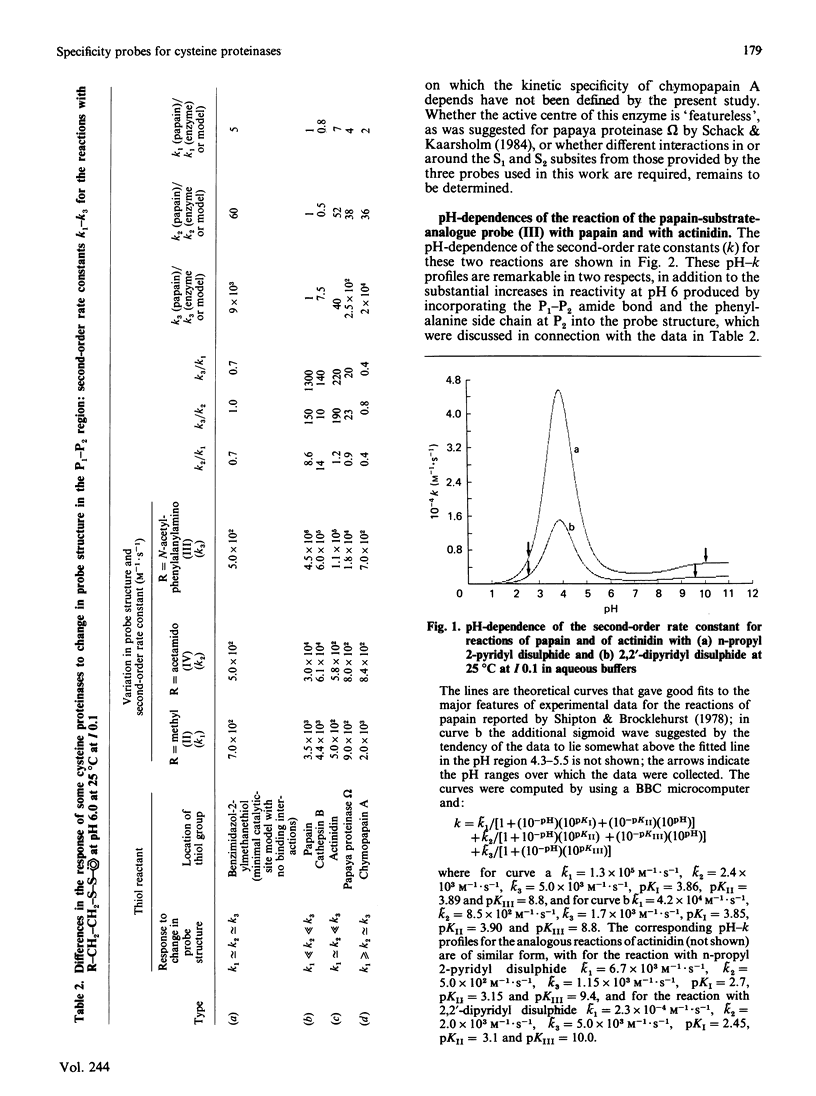
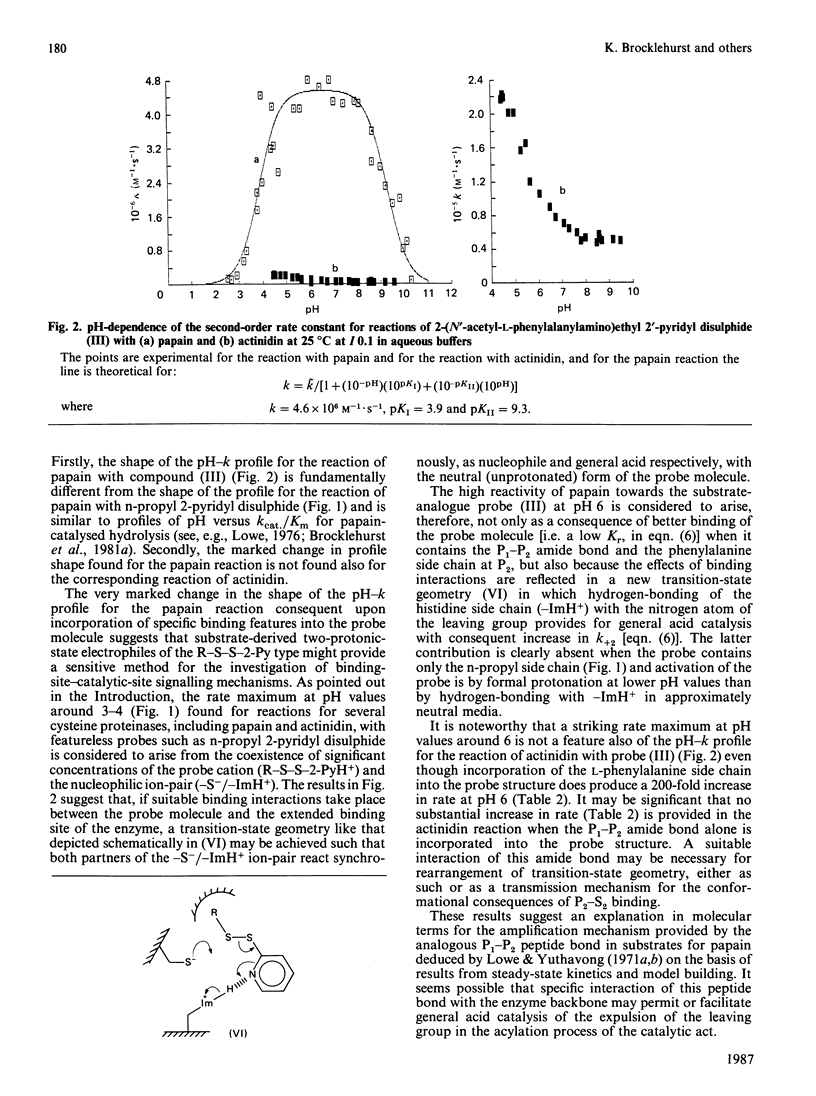
1. 2-(N'-Acetyl-L-phenylalanylamino)ethyl 2'-pyridyl disulphide [compound (III)] and 2-(acetamido)ethyl 2'-pyridyl disulphide [compound (IV)] were synthesized by acylation of the common intermediate, 2-aminoethyl 2'-pyridyl disulphide, to provide examples of chromogenic thiol-specific substrate-derived two-protonic-state electrophilic probe reagents. These two reagents, together with n-propyl 2-pyridyl disulphide [compound (II)], provide structural variation in the non-pyridyl part of the molecule from a simple hydrocarbon side chain in compound (II) to a P1-P2 amide bond in compound (IV) and further to both a P1-P2 amide bond and a hydrophobic side chain (of phenylalanine) at P2 as a potential occupant of S2 subsites. 2. These disulphides were used as reactivity probes to investigate specificity and binding-site-catalytic-site signalling in a number of cysteine proteinases by determining (a) the reactivity at pH 6.0 at 25 degrees C at I 0.1 of compound (III) (a close analogue of a good papain substrate) towards 2-mercaptoethanol, benzimidazol-2-ylmethanethiol [compound (V), as a minimal catalytic-site model], chymopapains B1-B3, chymopapain A, papaya proteinase omega, actinidin, cathepsin B and papain, (b) the effect of changing the structure of the probe as indicated above on the reactivities of compound (V) and of the last five of these enzymes, and (c) the forms of pH-dependence of the reactivities of papain and actinidin towards compound (III). 3. The kinetic data suggest that reagents of the type investigated may be sensitive probes of molecular recognition features in this family of enzymes and are capable not only of detecting differences in binding ability of the various enzymes but also of identifying enzyme-ligand contacts that provide for binding-site-catalytic-site signalling mechanisms. 4. The particular value of this class of probe appears to derive from the possibility of activating the 2-mercaptopyridine leaving group not only by formal protonation, as was recognized previously [see Brocklehurst (1982) Methods Enzymol. 87C, 427-469], but also by hydrogen-bonding to the pyridyl nitrogen atom when the appropriate geometry in the catalytic site is provided by enzyme-ligand contacts involving the non-pyridyl part of the molecule.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines B. S., Brocklehurst K. A necessary modification to the preparation of papain from any high-quality latex of Carica papaya and evidence for the structural integrity of the enzyme produced by traditional methods. Biochem J. 1979 Feb 1;177(2):541–548. doi: 10.1042/bj1770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K., Carey P. R., Jarvis M., Salih E., Storer A. C. Chymopapain A. Purification and investigation by covalent chromatography and characterization by two-protonic-state reactivity-probe kinetics, steady-state kinetics and resonance Raman spectroscopy of some dithioacyl derivatives. Biochem J. 1986 Jan 1;233(1):119–129. doi: 10.1042/bj2330119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K. Characterization of papaya peptidase A as a cysteine proteinase of Carica papaya L. with active-centre properties that differ from those of papain by using 2,2'-dipyridyl disulphide and 4-chloro-7-nitrobenzofurazan as reactivity probes. Use of two-protonic-state electrophiles in the identification of catalytic-site thiol groups. Biochem J. 1982 Jul 1;205(1):205–211. doi: 10.1042/bj2050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. N., Boland M. J., Calder P. C., Hardman M. J. The specificity of actinidin and its relationship to the structure of the enzyme. Biochim Biophys Acta. 1980 Nov 6;616(1):30–34. doi: 10.1016/0005-2744(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. PH-dependence of the steady-state rate of a two-step enzymic reaction. Biochem J. 1976 Apr 1;155(1):61–70. doi: 10.1042/bj1550061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. The pH-dependence of second-order rate constants of enzyme modification may provide free-reactant pKa values. Biochem J. 1977 Dec 1;167(3):859–862. doi: 10.1042/bj1670859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E. A re-evaluation of the nomenclature of the cysteine proteinases of Carica papaya and a rational basis for their identification. Biochem J. 1983 Aug 1;213(2):559–560. doi: 10.1042/bj2130559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. Validity of a 'steady-state' treatment of inactivation kinetics. Eur J Biochem. 1979 Jan 15;93(2):383–385. doi: 10.1111/j.1432-1033.1979.tb12834.x. [DOI] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. Proteinase-catalyzed synthesis of peptide bonds. Adv Enzymol Relat Areas Mol Biol. 1982;53:239–306. doi: 10.1002/9780470122983.ch7. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. The intrinsic pKa-values of functional groups in enzymes: improper deductions from the pH-dependence of steady-state parameters. CRC Crit Rev Biochem. 1976 Nov;4(2):165–173. doi: 10.3109/10409237609105457. [DOI] [PubMed] [Google Scholar]
- Little G., Brocklehurst K. Kinetics of the reversible reaction of papain with 5,5'-dithiobis-(2-nitrobenzoate) dianion: evidence for nucleophilic reactivity in the un-ionized thiol group of cysteine-25 and for general acid catalysis by histidine-159 of the reaction of the 5-mercapto-2-nitrobenzoate dianion with the papain-5-mercapto-2-nitrobenzoate mixed disulphide. Biochem J. 1972 Jun;128(2):475–477. doi: 10.1042/bj1280475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. pH-dependence and structure-activity relationships in the papain-catalysed hydrolysis of anilides. Biochem J. 1971 Aug;124(1):117–122. doi: 10.1042/bj1240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack P., Kaarsholm N. C. Subsite differences between the active centres of papaya peptidase A and papain as revealed by affinity chromatography. Purification of papaya peptidase A by ionic-strength-dependent affinity adsorption on an immobilized peptide inhibitor of papain. Biochem J. 1984 May 1;219(3):727–733. doi: 10.1042/bj2190727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968 Sep 6;32(5):898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brocklehurst K. Benzofuroxan as a thiol-specific reactivity probe. Kinetics of its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem J. 1977 Dec 1;167(3):799–810. doi: 10.1042/bj1670799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Chemical evidence for the pH-dependent control of ion-pair geometry in cathepsin B. Benzofuroxan as a reactivity probe sensitive to differences in the mutual disposition of the thiolate and imidazolium components of cysteine proteinase catalytic sites. Biochem J. 1986 Aug 15;238(1):103–107. doi: 10.1042/bj2380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Natural structural variation in enzymes as a tool in the study of mechanism exemplified by a comparison of the catalytic-site structure and characteristics of cathepsin B and papain. pH-dependent kinetics of the reactions of cathepsin B from bovine spleen and from rat liver with a thiol-specific two-protonic-state probe (2,2'-dipyridyl disulphide) and with a specific synthetic substrate (N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide). Biochem J. 1984 Sep 15;222(3):805–814. doi: 10.1042/bj2220805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]


