Abstract
The protoporphyrinogen-oxidizing enzyme from Triton X-100 extracts of the mitochondrial and etioplast fractions of etiolated barley was purified by using ion-exchange and hydroxyapatite chromatography. The purified enzyme from both organelle fractions exhibited a Km of 5 microM and was labile to mild heat and acidification. The pH optimum (5-6) and the substrate-specificity (mesoporphyrinogen was oxidized as rapidly as protoporphyrinogen) revealed properties very different from the protoporphyrinogen-oxidizing enzyme of rat liver or yeast mitochondria, which is specific for protoporphyrinogen as substrate. The purest fractions showed a polypeptide band corresponding to an Mr of approx. 36,000 on SDS/polyacrylamide-gel electrophoresis. This is the first purification and characterization of the enzyme from a plant, and indicates no readily detectable differences between the enzyme isolated from mitochondrial or etioplast fractions, although only the latter organelle has the capacity for both haem and chlorophyll synthesis.
Full text
PDF
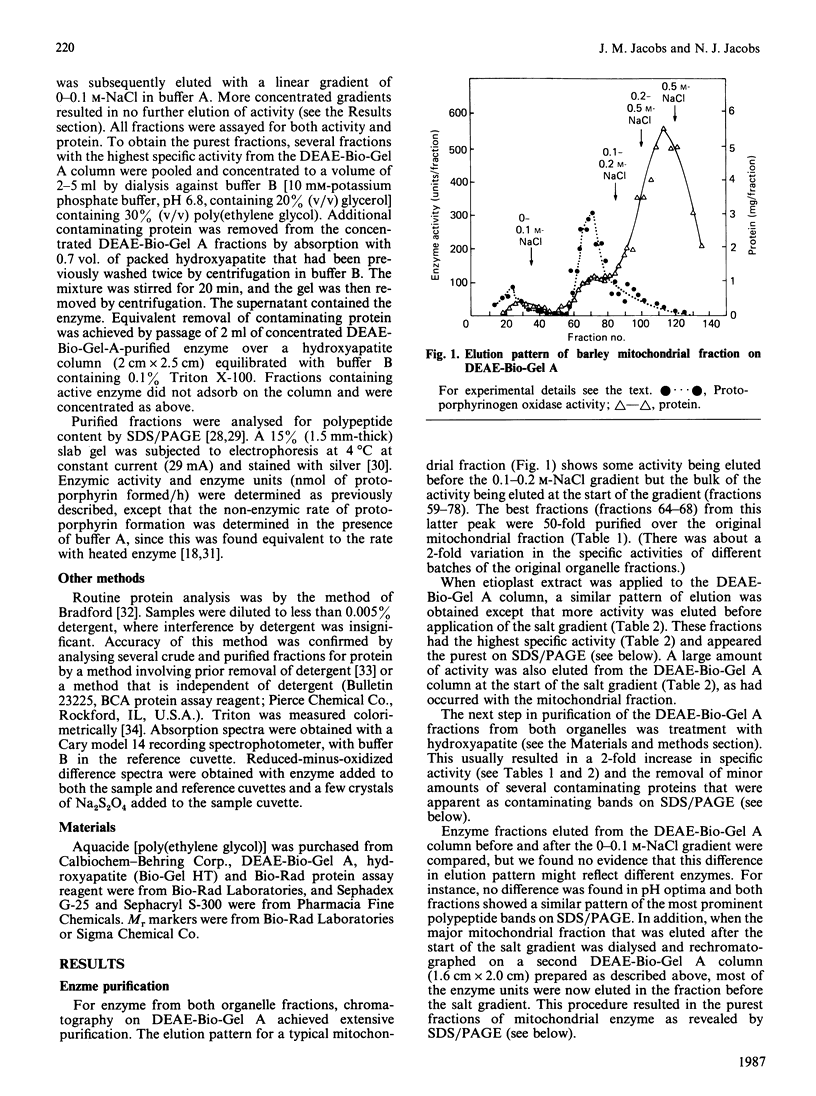
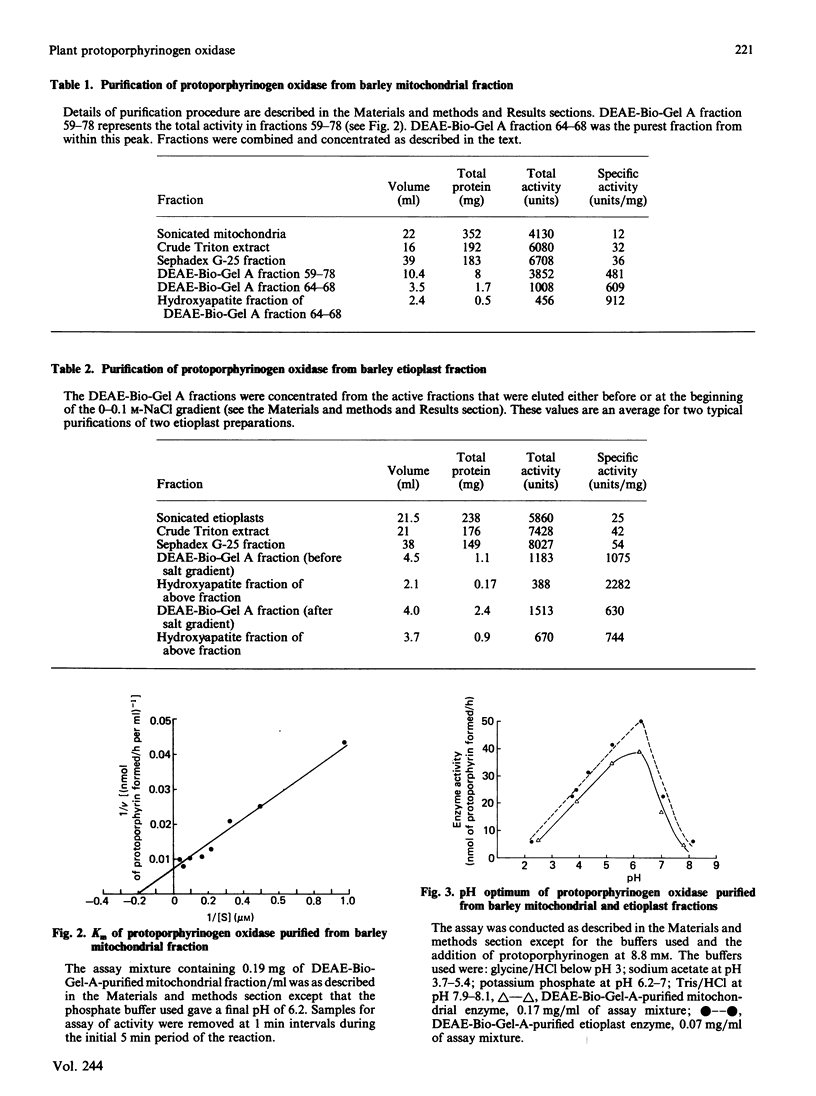
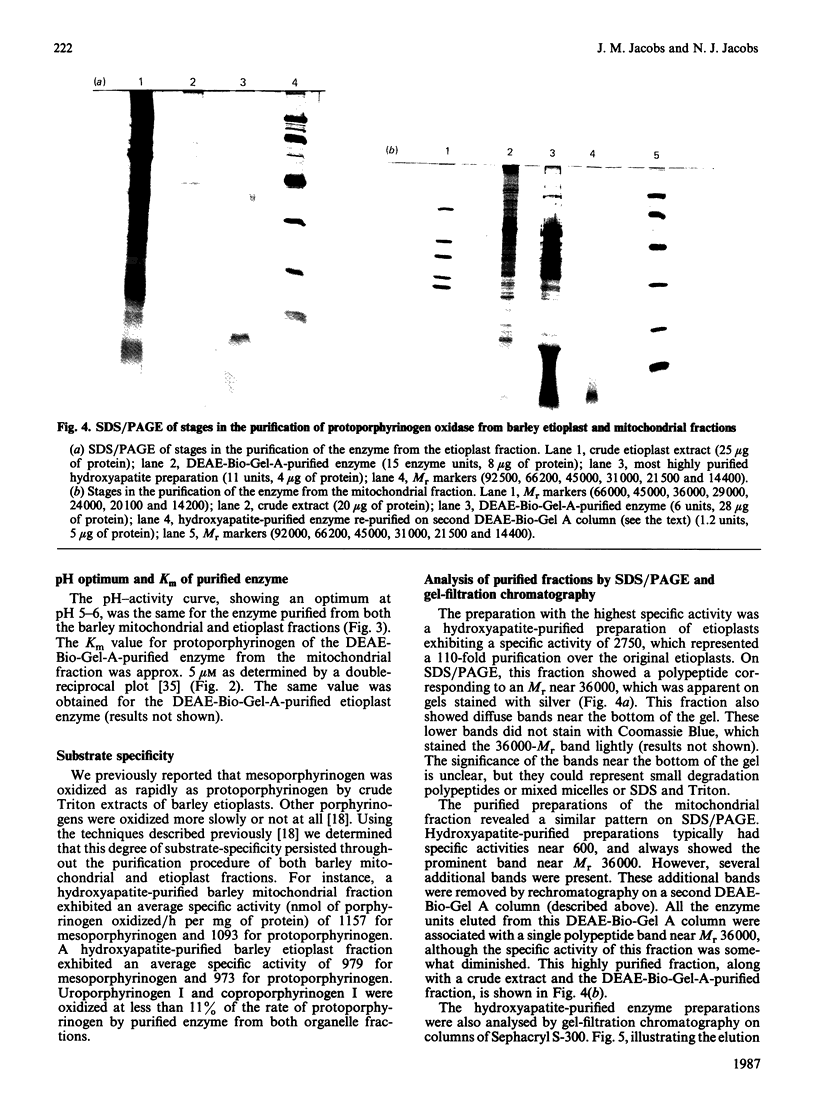
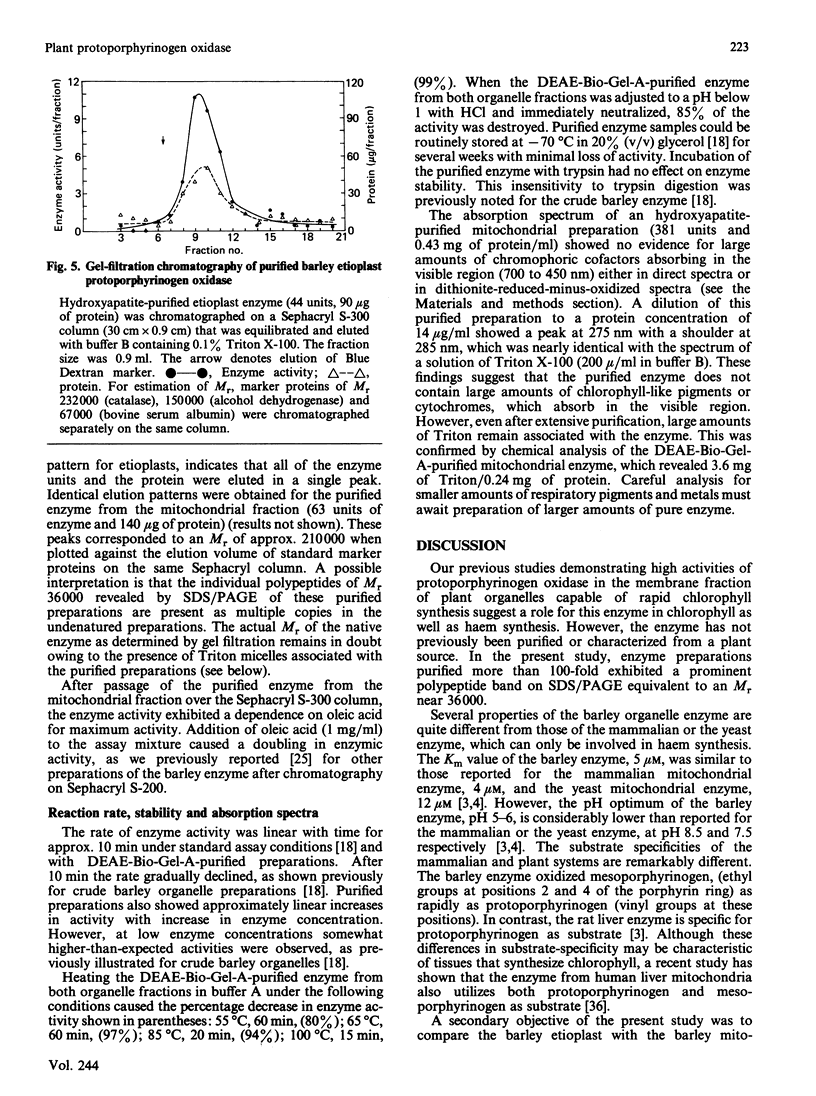

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battersby A. R., McDonald E., Redfern J. R., Staunton J., Wightman R. H. Biosynthesis of porphyrins and related macrocycles. Part V. Structural integrity of the type III porphyrinogen macrocycle in an active biological system; studies on the aromatisation of protoporphyrinogen-IX. J Chem Soc Perkin 1. 1976;(3):266–273. doi: 10.1039/p19760000266. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. The enzymatic defect in variegate prophyria. Studies with human cultured skin fibroblasts. N Engl J Med. 1980 Apr 3;302(14):765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- Camadro J. M., Abraham N. G., Levere R. D. Kinetic properties of the membrane-bound human liver mitochondrial protoporphyrinogen oxidase. Arch Biochem Biophys. 1985 Oct;242(1):206–212. doi: 10.1016/0003-9861(85)90494-1. [DOI] [PubMed] [Google Scholar]
- Camadro J. M., Urban-Grimal D., Labbe P. A new assay for protoporphyrinogen oxidase - evidence for a total deficiency in that activity in a heme-less mutant of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Jun 15;106(3):724–730. doi: 10.1016/0006-291x(82)91771-5. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., da Silva V., Grandchamp B., Nordmann Y. The mitochondrial location of protoporphyrinogen oxidase. Eur J Biochem. 1985 Jun 3;149(2):431–435. doi: 10.1111/j.1432-1033.1985.tb08943.x. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Nordmann Y. The inherited enzymatic defect in porphyria variegata. Hum Genet. 1981;58(4):425–428. doi: 10.1007/BF00282829. [DOI] [PubMed] [Google Scholar]
- Garewal H. S. A procedure for the estimation of microgram quantities of triton X-100. Anal Biochem. 1973 Aug;54(2):319–324. doi: 10.1016/0003-2697(73)90359-x. [DOI] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Games D. E., Couch P., Jackson J. R., Belcher R. B., Smith S. G. Conversion of coproporphyrinogen 3 to protoporphyrin IX. Enzyme. 1974;17(1):81–87. doi: 10.1159/000459311. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J., De Maggio A. E. Protoporphyrinogen oxidation in chloroplasts and plant mitochondria, a step in heme and chlorophyll synthesis. Arch Biochem Biophys. 1982 Oct 1;218(1):233–239. doi: 10.1016/0003-9861(82)90341-1. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J. Effect of unsaturated fatty acids on protoporphyrinogen oxidation, a step in heme and chlorophyll synthesis in plant organelles. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1157–1164. doi: 10.1016/s0006-291x(84)80254-5. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J. Protoporphyrinogen oxidation, an enzymatic step in heme and chlorophyll synthesis: partial characterization of the reaction in plant organelles and comparison with mammalian and bacterial systems. Arch Biochem Biophys. 1984 Feb 15;229(1):312–319. doi: 10.1016/0003-9861(84)90157-7. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Assay for enzymatic protoporphyrinogen oxidation, a late step in heme synthesis. Enzyme. 1982;28(2-3):206–219. doi: 10.1159/000459103. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Fumarate as alternate electron acceptor for the late steps of anaerobic heme synthesis in Escherichia coli. Biochem Biophys Res Commun. 1975 Jul 8;65(1):435–441. doi: 10.1016/s0006-291x(75)80112-4. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Microbial oxidation of protoporhydrinogen, an intermediate in heme and chlorphyll biosynthesis. Arch Biochem Biophys. 1979 Oct 15;197(2):396–403. doi: 10.1016/0003-9861(79)90261-3. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Nitrate, fumarate, and oxygen as electron acceptors for a late step in microbial heme synthesis. Biochim Biophys Acta. 1976 Oct 13;449(1):1–9. doi: 10.1016/0005-2728(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Protoporphyrinogen oxidation in Rhodopseudomonas spheroides, a step in heme and bacteriochlorophyll synthesis. Arch Biochem Biophys. 1981 Oct 1;211(1):305–311. doi: 10.1016/0003-9861(81)90458-6. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Quinones as hydrogen carriers for a late step in anaerobic heme biosynthesis in Escherichia coli. Biochim Biophys Acta. 1978 Dec 18;544(3):540–546. doi: 10.1016/0304-4165(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Klemm D. J., Barton L. L. Oxidation of protoporphyrinogen in the obligate anaerobe Desulfovibrio gigas. J Bacteriol. 1985 Oct;164(1):316–320. doi: 10.1128/jb.164.1.316-320.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little H. N., Jones O. T. The subcellular loclization and properties of the ferrochelatase of etiolated barley. Biochem J. 1976 May 15;156(2):309–314. doi: 10.1042/bj1560309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- Poulson R., Whitlow K. J., Polglase W. J. Catabolite repression of protoporhyrin IX biosynthesis in Escherichia coli K-12. FEBS Lett. 1976 Mar 1;62(3):351–353. doi: 10.1016/0014-5793(76)80092-0. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Protochlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):24–32. doi: 10.1104/pp.47.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]



