Abstract
Recombinant canarypox virus vectors containing human immunodeficiency virus type 1 (HIV-1) sequences are promising vaccine candidates, as they replicate poorly in human cells. However, when delivered intramuscularly the vaccines have induced inconsistent and in some cases transient antigen-specific cytotoxic T-cell (CTL) responses in seronegative volunteers. An attractive way to enhance these responses would be to target canarypox virus to professional antigen-presenting cells such as dendritic cells (DCs). We studied (i) the interaction between canarypox virus and DCs and (ii) the T-cell responses induced by DCs infected with canarypox virus vectors containing HIV-1 genes. Mature and not immature DCs resisted the cytopathic effects of canarypox virus and elicited strong effector CD8+ T-cell responses from chronically infected HIV+ individuals, e.g., cytolysis, and secretion of gamma interferon (IFN-γ) and β-chemokines. Furthermore, canarypox virus-infected DCs were >30-fold more efficient than monocytes and induced responses that were comparable to those induced by vaccinia virus vectors or peptides. Addition of exogenous cytokines was not necessary to elicit CD8+ effector cells, although the presence of CD4+ T cells was required for their expansion and maintenance. Most strikingly, canarypox virus-infected DCs were directly able to stimulate HIV-specific, IFN-γ-secreting CD4 helper responses from bulk as well as purified CD4+ T cells. Therefore, these results suggest that targeting canarypox virus vectors to mature DCs could potentially elicit both anti-HIV CD8+ and CD4+ helper responses in vivo.
Current antiviral treatments consisting of highly active antiretroviral therapy (HAART) have made a major impact on reducing mortality due to human immunodeficiency virus (HIV) infection (39). However, HAART does not reduce viral loads in all patients (43), and even in patients with no detectable plasma viremia, latent reservoirs of HIV persist for prolonged periods (10, 23, 24, 25, 28, 59, 61). Recent studies estimate that more than 60 years of HAART would be required to eradicate the virus in the latent reservoirs (9). As antiviral drugs are too expensive to be widely used in developing countries, the development of anti-HIV vaccines is of great urgency.
There is strong evidence supporting a role of cytotoxic T lymphocytes (CTLs) in the containment of HIV replication. In early HIV infection, the appearance of CTLs correlates with control of viremia and reduction of symptoms (34). In chronic infection, major histocompatibility complex (MHC) tetramer studies show an inverse correlation between CTL effectors and low viral loads (40). In late infection, the loss of anti-HIV CTL responses correlates with higher viral loads and progression of disease (32). Individuals with multiple exposures to HIV but who remain uninfected show anti-HIV CTL responses in some cases (47). Finally, CD8+ CTLs have been shown to be critically involved in the control of simian immunodeficiency virus in macaques, the best model of HIV infection in humans (29, 49).
HIV-specific CD4+ T cells also contribute to immune resistance toward HIV. Individuals who maintain a very low viral load and do not progress to disease have vigorous HIV-specific CD4+ T-cell responses (44, 46), along with strong and broad anti-HIV CTL responses (31). Further studies have supported an association between robust HIV type 1 (HIV-1)-specific CTLs and strong helper cell responses (30, 58). A drop in HIV-specific CD4+ T cells leads to a decline in anti-HIV CTL levels and more rapid disease progression (31, 44, 46). Presumably, effective anti-HIV vaccines will need to elicit CD4+ helper as well as CD8+ CTL responses in order to maintain effective CTL function.
Several approaches are being taken to elicit anti-HIV CTL responses using vaccine formulations (reviewed in reference 37). A promising approach entails canarypox virus vectors. Canarypox virus undergoes abortive replication in mammalian cells (42, 55). Recombinant genes are controlled by early promoters in canarypox virus and expressed before the block in replication (42, 55). Canarypox vaccines have an excellent safety profile in phase 1 trials, and their effectiveness against a variety of infectious agents has been demonstrated in both animals and humans (42, 54). Canarypox virus vectors containing HIV-1 genes (can-HIV vectors) have been reported to elicit specific CTL responses in uninfected volunteers when administered intramuscularly (5, 11, 17, 19, 21, 22). However, the responses have been intermittent and inconsistent, sometimes requiring the addition of cytokines in vitro for detection (26). To increase the magnitude and durability of these responses, it may be critical to target these vectors to potent antigen-presenting cells (APCs), namely, dendritic cells (DCs) (4, 51).
In this study, we characterized the interaction between canarypox virus and DCs at different stages of their development. We found that mature DCs infected with can-HIV stimulated IFN-γ- and β-chemokine-producing and cytolytic CD8+ effector cells in vitro from chronically infected individuals. These responses, which are induced only by DCs and not other APCs, were readily detectable in the absence of repetitive stimulation or exogenous cytokines. Strikingly, canarypox virus-infected DCs also expanded HIV-specific CD4+ T cells in culture. These CD4+ T-cell responses were essential for the development of anti-HIV CD8+ CTLs. Our results reveal for the first time that canarypox virus has the potential to stimulate both CD4+ and CD8+ arms of the anti-HIV immune response. They support the use of canarypox virus as a vaccine vector which has the potential to elicit virus-specific CD4+ T-cell help for the induction and maintenance of CTL responses to HIV-1.
MATERIALS AND METHODS
Culture medium.
RPMI 1640 medium with 10 mM HEPES, 5 mM l-glutamine, 20 μg of gentamicin per ml, and 1% human plasma, 5% heat-inactivated human serum, or 10% fetal calf serum was used.
Human subjects.
Patients, recruited through the Rockefeller University clinical research center, signed informed consents approved by the Institutional Review Board. All nine individuals were 37- to 47-year-old males chronically infected with HIV-1 (duration of infection ranged from 4 to 9 years) who had CD4 counts ranging from 75 to 633/μl and plasma viremia levels which ranged from undetectable to 192 × 103/ml by the Roche Ultrasensitive PCR kit. Six of the nine individuals were on HAART, and two were on therapy intermittently due to noncompliance. Seronegative individuals served as controls. Three patients expressed HLA A∗0201.
APCs.
Buffy coats from uninfected individuals or 60 to 80 ml of blood from HIV-1+ patients were sources of peripheral blood mononuclear cells (PBMCs). Mononuclear cells, enriched or depleted of T cells, were obtained by rosetting PBMCs with neuraminidase-treated sheep erythrocytes (6). Immature DCs were generated from the T-cell-depleted fractions after supplementation with recombinant human interleukin-4 (IL-4 1,000 U/ml; Schering Plough Corporation, Kenilworth, N.J.) and recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 IU/ml; Immunex Corporation, Seattle, Wash.) every other day. To generate mature DCs, nonadherent immature DCs were transferred to new plates on day 6 and incubated for 2 days in monocyte conditioned medium (MCM; 50%, vol/vol) prepared as previously described (7). HIV-negative donors were used as a source of monocytes for preparing the MCM.
Virus stocks.
The recombinant WR vaccinia viruses used were vP1170 WR-eco gpt (parental), vP1287 gag(IIIB), vP1288 pol(IIIB), vP1218 nef(MN), and vP1286 env gp120 TM(MN), containing HIV-1 clade B gag, pol, nef, and env genes. Canarypox virus vectors were ALVAC (parental) and vCP300 encoding HIV-1 gp120(MN) and transmembrane anchor regions of gp41(LAI), Gag(LAI), and protease(LAI); Pol(LAI) CTL domains (residues 172 to 219, 325 to 383, and 461 to 519); and Nef(BRU) CTL domains (residues 66 to 147 and 182 to 206). Canarypox virus (5 to 10 PFU/cell or vaccinia virus (1 to 2 PFU/cell) was used to infect APCs. James Tartaglia and William I. Cox (Virogenetics Corporation, Troy, N.J.) provided the titered doses of virus stocks.
Fluorescence-activated cell sorting (FACS) analysis.
Monoclonal antibody (MAb) 183, directed to HIV p24 protein, was kindly provided by Melissa Pope. Phycoerythrin (PE)-conjugated HLA DR, CD14, CD25, and isotype-matched controls (Becton Dickinson, Montainview, Calif.), CD86 (PharMingen, San Diego, Calif.), CD83 (Immunotech, Coulter Corporation, Hialeah, Fla.), and PE-conjugated goat anti-mouse immunoglobulin G (IgG; TAGO, Burlingame, Calif.) were used for phenotyping. For surface staining, cells were phenotyped with the above panel of MAbs using a FACScan. For intracellular staining, cells were fixed with 4% paraformaldehyde and permeabilized with 1% saponin (6). Antibody to HIV p24 protein was added for 30 min, cells were washed, and secondary PE-conjugated goat-anti mouse IgG was added for 30 min prior to FACScan analysis.
Viability.
In addition to trypan blue exclusion, apoptosis and necrosis were assessed by staining with fluorescein isothiocyanate (FITC)-annexin V and propidium iodide, using an Early Apoptosis detection kit (Kayima Biomedical Company, Seattle, Wash.).
IFN-γ ELISPOT assays.
PBMCs, monocytes, or DCs were infected with poxviruses, and IFN-γ enzyme-linked immunospot (ELISPOT) assays were carried out as described elsewhere (35). In brief, poxvirus-infected or uninfected cells were added together with T cells (1 × 105 to 2 × 105/well) to 96-well plates precoated with IFN-γ antibody (Mabtech, Stockholm, Sweden) for 16 to 24 h. After washing, a second biotinylated anti-IFN-γ antibody (Mabtech) was added followed by avidin-bound biotinylated horseradish peroxidase H (Vector Laboratories, Burlingame, Calif.) to develop the spots. ELISPOT assays were also used to assess the expansion of antigen-specific T cells over time. T cells were cocultured for 7 days with DCs infected with canarypox virus control (can-ctl) or can-HIV. ELISPOTs were then elicited in responding T cells by restimulation with antigen-pulsed APCs. The latter consisted of monocytes infected with vaccinia virus or canarypox virus vectors or pulsed with 5 μg of either HIV p24 or control protein (Protein Science, Meriden, Conn.) per ml. T cells and monocytes were used at a ratio of 1:1. Cells stimulated with phytohemagglutinin were used as a positive control, and T cells, DCs, or monocytes alone were negative controls. Responses were counted as positive if a minimum of 10 spot-forming cells (SFC) per 2 × 105 cells were detected after the control was subtracted, and if the numbers of spots were at least twice those in the negative control wells.
RANTES detection.
DCs were infected with either can-ctl or can-HIV and cocultured with autologous T cells at a DC-to-T cell (DC:T) ratio of 1:30. After 6 to 7 days, the supernatants of cultures with HIV-specific CTLs were tested for RANTES using an enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems, Minneapolis, Minn.).
CTL induction.
Monocytes and mature DCs (107 cells/ml) were infected with can-ctl (ALVAC) or can-HIV (vCP300) at a multiplicity of infection (MOI) of 10, or infected with vaccinia virus at MOIs of 1 to 2.5, for 1 h at 37°C. The cells were washed twice and added to enriched T cells. Where indicated, DCs were pulsed with the HLA A∗0201-restricted Pol peptide ILKEPVHGV (10 μg/ml) for 2 h at room temperature. The T-cell-enriched fraction was obtained from sheep erythrocyte rosetted cells by depletion of NK cells with anti-CD56 (PharMingen) and sheep anti-mouse magnetic beads (Dynal, Lake Success, N.Y.). In some experiments, T cells were further purified into CD8+ and CD4+ T-cell fractions using magnetic beads (Miltenyi Biotech, Auburn, Calif.); 2 × 106 T-cells were cultured with APCs at a ratio of 10:1 (unless otherwise indicated) in 24-well plates for 7 days.
Chromium release assay.
After 7 days, effector cells in the DC-T cell cocultures were harvested, counted, and plated in graded doses in 96-well plates. B-lymphoblastoid cell lines (BLCLs) generated from each patient served as targets. The BLCLs were infected with recombinant vaccinia virus vectors as described above and incubated with 4 μCi of Na51CrO4 for 1 h. Alternatively, T2, an HLA A∗0201+ class II− and transporter-associated protein (TAP)-deficient cell line, was used as a target. T2 cells were pulsed with the HLA A∗0201-restricted influenza virus matrix peptide GILGFVFTL (negative control peptide) or HLA A∗0201-restricted HIV-1 Gag SLYNTVATL and Pol ILKEPVHGV peptides at 10 μg/ml for 1 h and then labeled with Na51CrO4 as described above. Target cells were added to effector cells at effector-to-target cell (E:T) ratios of 30:1 to 10:1. After 5 to 6.5 h, the assay mixtures were harvested. Two steps were taken to calculate HIV-1 antigen-specific lysis. We first calculated the percent specific lysis for each stimulating APC population (e.g., can-ctl- or can-HIV-infected DCs) using the formula (ER − SR)/(TR − SR), where ER represents the release in the experimental sample, SR is spontaneous release, and TR is total release. We then deducted any nonspecific lysis obtained with DCs pulsed with control vectors or peptides from that obtained by DCs pulsed with HIV-1 antigen-expressing vectors or peptides. This value is referred to as HIV antigen-specific lysis.
RESULTS
Interactions of canarypox virus vectors and DCs.
In previous studies we found that poxvirus vectors profoundly affected DC function (18). For example, vaccinia virus induces extensive apoptosis of immature DCs, inhibits their maturation, and diminishes their T-cell-stimulating capacity. In contrast, mature DCs are relatively resistant to these adverse outcomes. Therefore, we investigated the consequences of canarypox virus infection on DCs in terms of cytopathicity, maturation effects, and extent and durability of HIV protein expression.
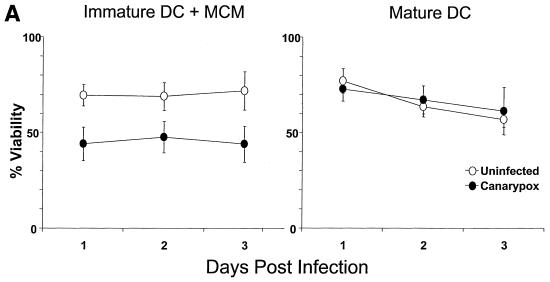
We first compared the effects of canarypox virus infection on DC viability at two distinct stages of development. Immature DCs, akin to tissue resident DCs, can be derived in vitro from monocytes following culture in GM-CSF and IL-4. These APCs are highly efficient at antigen capture but far less able to activate T cells (4). Mature DCs are generated from immature DCs after the addition of maturation stimuli such as a MCM, lipopolysaccharide or CD40 ligand (CD40L). Following maturation, DCs downregulate antigen capture, upregulate MHC and costimulatory molecules, express the maturation-associated markers CD83 (62) and DC-LAMP (14), and acquire potent T-cell-stimulating capacity (4). We infected either immature or mature DCs with can-HIV or can-ctl. In the former case, the immature DCs were exposed to MCM immediately after infection to induce maturation. If the DCs were immature at the time of infection, there was a rapid and significant decrease in viability as assessed by trypan blue exclusion. The effect was more rapid than with vaccinia virus, apparent after only 1 day postinfection (Fig. 1A). Mature DCs resisted the cytopathic effect to a great extent, as in the case of vaccinia virus (18). To analyze the mechanism of cytopathicity, DCs were stained with FITC-annexin V, a marker of early apoptosis (33, 56), and propidium iodide. Up to 60% of infected immature DCs were already apoptotic or dead at 1 day postinfection, compared to only 15 to 20% infected mature DCs, when one discounts uninfected control values (Fig. 1B). The apoptotic effect was clearly induced by canarypox virus and not HIV genes, as we used the parental vector for these experiments. Furthermore, similar results were obtained with can-HIV. Unlike vaccinia virus, canarypox virus did not inhibit DC maturation when MCM was added to canarypox virus-infected immature DCs (data not shown).
FIG. 1.
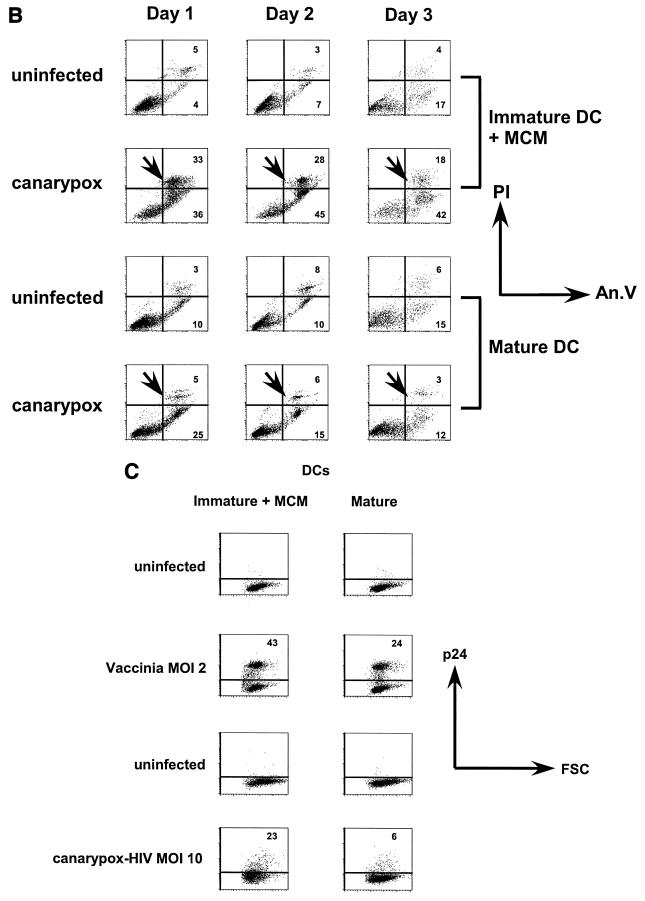
Interaction of recombinant poxvirus with DCs. (A) Immature and mature DCs were uninfected or infected with vac-gag (MOI of 2) or can-HIV (MOI of 10). The immature DCs were exposed to MCM immediately following infection. The percentage of live cells, as determined by trypan blue exclusion, is shown in immature DCs plus MCM (left) and mature DCs (right) at different time points after infection. The mean and standard error of three experiments are shown. (B) Immature DCs were uninfected or infected with canarypox virus, after which MCM was added. Mature DCs were infected or uninfected in like manner. At multiple time points after infection, the extent of apoptosis and necrosis was determined by staining the cells with FITC-annexin V (An.V) and propidium iodide (PI). Results shown are representative of three experiments. (C) Immature and mature DCs were uninfected or infected with vac-gag (MOI of 2) or can-HIV (MOI of 10) as for panel A; 24 h later, the cells were permeabilized and stained with a MAb against HIV-1 p24 protein. Anti-IgG1 antibody was the isotype control used to set the horizontal limit of background staining. The gates were set to exclude dead cells. One representative experiment of eight is shown. In panels B and C, the y axis is set on a logarithmic scale and the percentages of cells are indicated in the corresponding gates.
We next compared the levels of HIV-1 protein expression in DCs following infection with can-HIV and a vaccinia virus construct containing the gag gene (vac-gag). As above, we infected either immature or mature DCs, and in the former case, the immature DCs were exposed to MCM immediately after infection to induce maturation. The cells were stained to detect p24 expressed by the gag gene as a measure of the degree of infection (Fig. 1C; Table 1). Vaccinia virus vectors encoding Gag induced higher frequencies of p24+ cells in immature DCs (67% ± 21%) and mature DCs (44% ± 20%) compared to can-HIV (15% ± 11% in immature DCs and 7% ± 6% in mature DCs). Maturation reduced the frequency of p24-expressing DCs, consistent with our previous observations that mature DCs are generally more resistant to poxvirus infection. Nevertheless, p24 expression in mature DCs was sustained over 3 days in culture (data not shown). We found MOIs of 5 to 10 to be optimal for canarypox virus-induced recombinant protein expression. Lower doses (MOIs of 1 to 2) resulted in even lower levels of p24 expression, whereas higher doses did not increase the levels significantly but compromised viability (not shown). These data are consistent with our recent studies of vaccinia viruses (18).
TABLE 1.
p24 expression in DCs infected with poxvirus vectorsa
| Vector | Immature DC + MCM
|
Mature DC
|
||
|---|---|---|---|---|
| % p24+ cells mean ± SD | No. of expts | % p24+ cells mean ± SD | No. of expts | |
| vac-ctl | 0 ± 0 | 7 | 0 ± 0 | 6 |
| vac-gag | 67 ± 21 | 8 | 44 ± 20 | 8 |
| can-ctl | 0 ± 0 | 5 | 0 ± 0 | 4 |
| can-HIV | 15 ± 11 | 11 | 7 ± 6 | 11 |
DCs were infected with vaccinia virus and canarypox virus vectors at MOIs of 2 to 5 and 5 to 10, respectively. In the case of immature DC, the cells were immature at the time of infection and MCM was added directly after the infection. One day later, cells were permeabilized, stained with anti-p24 antibody, and analyzed by FACS.
Although only low frequencies of mature DCs were infected with canarypox virus, their resistance to the virus's cytopathic effects, sustained protein expression, and potency as stimulators of T-cell responses prompted us to use them for all subsequent experiments.
Mature DCs, but not monocytes, induce strong HIV-1-specific CD8+ responses following infection with canarypox virus.
To ascertain whether canarypox virus-infected mature DCs could present HIV antigens to CD8+ T cells, we took advantage of a cohort of nine chronically infected HIV-1 individuals who had been previously characterized in our laboratory (see Materials and Methods). Patients were screened for HIV-specific CD8+ T-cell responses in fresh PBMCs by ELISPOT assay using vaccinia virus vectors encoding HIV genes (35). All individuals had responses to antigens derived from Pol, four had responses to both Pol and Gag antigens, and one had responses to antigens derived from Gag, Pol, Env, and Nef. The number of HIV-specific CD8+ T cells ranged from 20 to 225 in 200,000 PBMCs, i.e., an HIV-1 antigen-specific frequency of 1 in 600 to 10,000 PBMCs. We prepared DCs and monocytes from each of these individuals, infected them with can-HIV or can-ctl, and cocultured them with autologous T cells. The development of HIV-specific CD8+ T-cell responses was assessed by (i) cytolysis by chromium release assay, (ii) RANTES secretion by ELISA, and (iii) IFN-γ production by ELISPOT assay.
Induction of CTL.
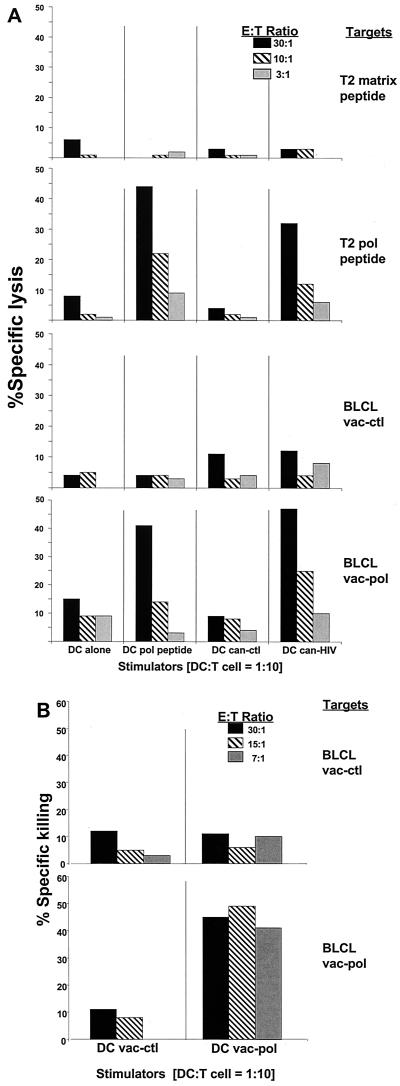
We first evaluated CTL responses in an HLA A∗0201+ patient (HVR) with known specificity to the pol-encoded epitope ILKEPVHGV (35). Mature DCs from this individual were either untreated or infected with can-ctl or can-HIV and then added to freshly isolated T cells for 7 days, during which no exogenous cytokines were added. The responses were compared to those elicited by DCs pulsed with specific peptide or vaccinia virus vectors. Effector CTLs were assessed by their cytolytic activity on 51Cr-labeled autologous BLCLs or T2 targets. These were infected with vaccinia virus vectors or pulsed with HLA A∗0201-restricted peptides, respectively. DCs infected with can-HIV stimulated peptide-specific responses that were comparable to those stimulated by DCs pulsed with peptide (28 versus 42% HIV-specific lysis, respectively, at an E:T ratio of 30:1 [Fig. 2A, top half). In both cases, the CTLs recognized endogenously presented antigens, as they lysed BLCL targets infected with vaccinia virus vectors encoding Pol antigens (26 versus 35% HIV-specific lysis for peptide versus can-HIV-pulsed DCs, respectively, at an E:T ratio of 30:1 [Fig. 2A, bottom half]). Surprisingly, the responses elicited by can-HIV-infected DCs were similar in magnitude to those induced by vac-pol-infected DCs, at least at the higher E:T ratio of 30:1 (Fig. 2B). The vac-pol vector was expected to be superior to canarypox virus as it infects >40% of DCs and contains the entire pol gene, while can-HIV contains only specific domains of pol (see Materials and Methods). In two additional individuals tested, can-HIV-infected DCs elicited CTL responses that were comparable to those induced by DCs infected with vaccinia virus vectors (data not shown).
FIG. 2.
DCs infected with can-HIV antigens induce CTL responses. (A) DCs generated from an HLA A∗0201+ individual (HVR) were either uninfected, pulsed with the HLA A∗0201-restricted Pol peptide (ILKEPVHGV), or infected with can-ctl or can-HIV. DCs were coincubated with autologous T cells for 7 days at DC:T ratios of 10:1, after which effectors were tested for cytolytic activity. Targets were T2 cells pulsed with an irrelevant influenza virus matrix peptide (T2 matrix peptide) or with the Pol peptide (T2 Pol peptide), and autologous BLCLs were infected with vac-ctl (BLCL vac-ctl) or vac-pol (BLCL vac-pol). E:T ratios of 30:1, 10:1 and 3:1, were tested. (B) DCs from the same individual were infected with vac-ctl or vac-pol at a MOI of 2 and cocultured with T cells. Cytolytic activity was measured after 7 days at E:T ratios of 30:1 to 7:1. Targets were autologous BLCLs infected with vac-ctl or vac-pol.
Using can-HIV-infected DCs, it was possible to detect significant HIV-specific CTL responses in five of seven patients tested by this assay, and in a reproducible fashion (Table 2). In these five patients, cytolytic activity was directed against one or more HIV antigens. In two patients (HVJ and HVW) who had demonstrable HIV-directed responses by ELISPOT assay, specific CTL activity was not detected, possibly due to high background responses to can-ctl. Notably, HIV-1-specific responses were not seen in seronegative volunteers (data not shown).
TABLE 2.
Canarypox virus-infected DCs elicit CD8+ CTL responses in chronically infected patientsa
| Patient | Age (yr) | No. of assays with HIV specific response/total | % HIV-specific lysis | Antigen specificity |
|---|---|---|---|---|
| HVRb | 38 | 3/3 | 35–62 | Pol |
| HVPb | 49 | 2/2 | 28–58 | Gag |
| HVM | 44 | 1/1 | 20–63 | Gag, Pol |
| HVC | 46 | 2/2 | 12–20 | Gag, Env, Nef |
| HVGb | 44 | 3/3 | 13–17 | Pol |
| HVJ | 41 | 0/2 | ND | NA |
| HVW | 42 | 0/1 | ND | NA |
Responder cells were T cells stimulated for 7 days with DCs and analyzed for cytotoxicity against 51Cr-labeled autologous BLCLs infected with vaccinia virus vectors encoding HIV antigens. HIV-specific lysis was obtained after subtracting both lysis against vaccinia virus antigens and lysis stimulated by canarypox virus antigens. Lysis above 10% was considered significant. NA, not applicable; ND, not done.
HLA A∗0201 positive.
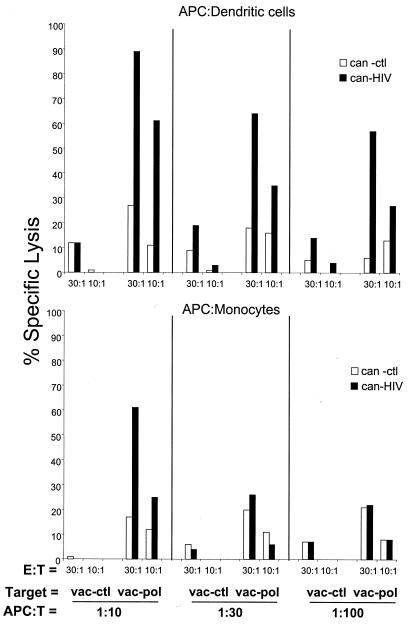
We next compared the immunostimulatory effect of DCs with a T-cell-depleted fraction of PBMCs consisting primarily of monocytes. DCs stimulated strong HIV-specific responses in individual HVR (62% specific lysis at an E:T ratio of 30:1 [Fig. 3, top]). These responses were maintained even at E:T ratios as low as 3:1 (data not shown). Furthermore, significant CTL responses could be elicited with even few DCs. For example, at DC:T ratios of 1:100, up to 56% specific lysis for Pol-derived antigens was obtained (Fig. 3, upper right). In contrast, monocytes were capable of inducing HIV-specific CTL responses only at an APC:T ratio of 1:10 and only at E:T ratios of 30:1 or greater (Fig. 3, bottom). Similar results were obtained with two additional subjects. Overall, these results show that DCs are substantially more potent than monocyte-enriched populations for the stimulation of anti-HIV-1 CTLs.
FIG. 3.
DCs are more potent than monocytes in inducing anti-HIV-CTLs. DCs or monocytes from individual HVR were infected with can-ctl or can-HIV and cocultured with autologous T cells at various APC:T ratios. Cytolytic activity was measured on day 7 using autologous BLCLs infected with vac-ctl or vac-pol as targets at E:T ratios of 30:1 and 10:1.
RANTES secretion.
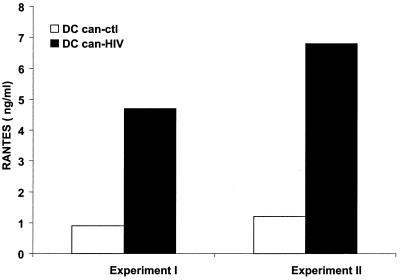
The β-chemokines RANTES, MIP-1α, and MIP-1β are the principal anti-HIV molecules secreted by CD8+ T cells. These chemokines may inhibit viral entry into CD4+ cells by binding to CCR5, the coreceptor of macrophagetropic HIV-1 (12, 57, 60). We measured the concentration of RANTES in supernatants from 7-day cocultures of T cells and can-HIV-infected DCs by ELISA. The results shown are from patient HVC, who was tested on two different occasions (Fig. 4). CD8+ T cells stimulated with can-HIV-infected DCs produced significant levels of RANTES and also lysed vac-gag-infected BLCLs (Table 2). In contrast, can-ctl-infected DCs failed to induce significant levels of RANTES or HIV-specific CTL activity. Similar data were obtained with two other patients (not shown). These data suggest that β-chemokine secretion correlates with the induction of HIV-specific CD8+ T-cell responses by canarypox virus-infected mature DCs.
FIG. 4.
Can-HIV-infected DCs elicit RANTES secretion. DCs from individual HVC were infected with can-ctl or can-HIV and cocultured with autologous T cells at an APC:T ratio of 1:30. After 6 to 7 days, HIV-specific cytolytic responses were obtained (Table 2), and the supernatants of such cultures were tested for the presence of the β-chemokine RANTES using an ELISA kit (R&D).
IFN-γ production.
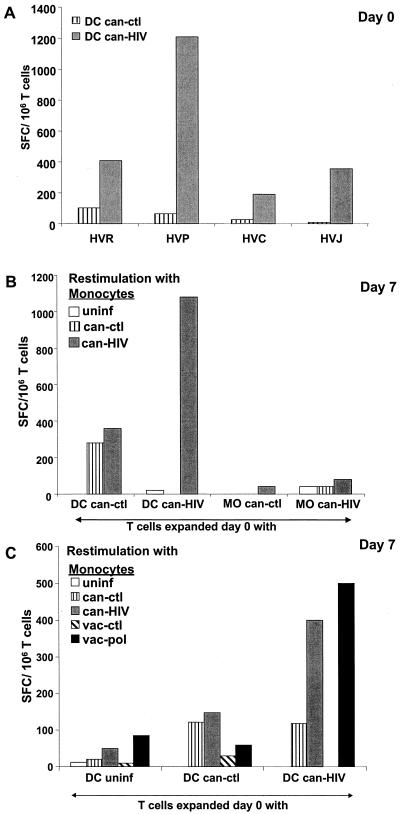
IFN-γ is a key antiviral cytokine produced by CD8+ effector cells. We determined whether can-HIV-infected DCs could elicit IFN-γ-producing CD8+ T cells from freshly isolated T cells of chronically infected individuals. In all of five subjects tested by ELISPOT assay, DCs induced significant levels of HIV-specific, IFN-γ-producing SFC within 24 h (range, 190 to 1,210 SFC/106 T cells at a DC:T ratio of 1:10 [Fig. 5A]). The responses were up to sixfold greater in magnitude than those induced by can-HIV-infected PBMCs, where primarily monocytes comprise the APCs (up to 30% of the PBMC fraction [35]).
FIG. 5.
Can-HIV-infected DCs elicit and expand IFN-γ-secreting cells. (A) Mature DCs from four HIV-1+ individuals were infected with can-ctl or can-HIV and added to freshly sampled autologous T cells at DC:T of 10:1. IFN-γ SFC were enumerated after 24 h by ELISPOT assay. (B) Cocultures of T cells and canarypox virus-infected DCs or monocytes from individual HVR were allowed to expand for 7 days. IFN-γ was then induced in the responding T cells by exposure to autologous monocytes uninfected or infected with can-ctl or can-HIV. uninf, uninfected. (C) T cells and canarypox virus-infected DCs from individual HVJ were cultured for 7 days. IFN-γ was then induced in the responding T cells by exposure to autologous monocytes uninfected or infected with can-ctl, can-HIV, vac-ctl, or vac-pol. In panels B and C, monocytes or T cells alone were additional controls, and these values were subtracted from experimental values.
Importantly, can-HIV-infected DCs also successfully expanded HIV-specific IFN-γ-producing T cells over several days of culture without the addition of exogenous cytokines. A representative example of seven experiments is shown in Fig. 5B. T cells from individual HVR were cocultured with can-HIV-infected DCs and tested for specificity on day 7 by ELISPOT assay. Specificity for HIV-1 antigens was assessed by restimulating the T cells for 24 h with autologous monocytes infected with can-ctl or can-HIV. One-day restimulation by poxvirus-infected APCs induces IFN-γ production from CD8+ T cells and not CD4+ T cells (35). At least a two- to threefold increase in HIV-specific SFC number was evident by this recall assay (compare HVR data in Fig. 5A and B). Although can-ctl-infected DCs induced significant numbers of SFCs compared to uninfected DCs, no HIV-specific responses were elicited by these cells. In contrast to DCs, monocytes failed to expand HIV-specific IFN-γ-producing CD8+ T cells over 7 days (Fig. 5B).
We next applied the recall ELISPOT assay to identify HIV-specific responses in individuals in whom CTL responses were not detected. For example, subject HVJ had a previously characterized Pol-specific CD8+ T-cell response by overnight ELISPOT assay, but we could not elicit HIV-specific cytolytic activity when his T cells were stimulated with can-HIV-infected DCs (Table 2). However, when recall ELISPOT assays were used, we were easily able to visualize the expansion of HIV-specific CD8 effectors from this individual. The responding cells also included Pol-specific effectors since they could be stimulated with vac-pol-infected monocytes (Fig. 5C). Thus, this assay allows one to detect specific responses that may be obscured in CTL assays. This disparity between different assays may be due to high cytolytic backgrounds from nonspecific NK cell responses. Alternatively, this subject may have HIV-specific CD8+ T cells that produce antiviral cytokines but are impaired in cytolytic function (2).
In summary, can-HIV-infected mature DCs have the capacity to elicit strong anti-HIV CD8+ effector cells which are characterized by their production of IFN-γ, β chemokines, and cytolytic activity.
Expansion of CD8+ effectors by canarypox virus requires CD4+ T-cell help.
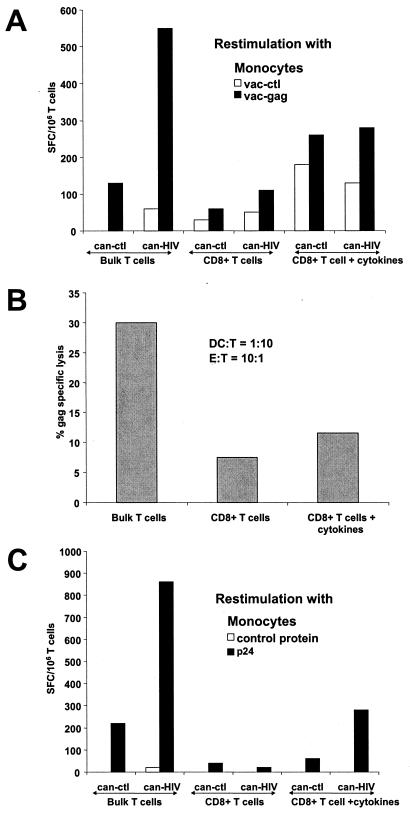
We next assessed whether CD4+ T cells were required for expanding HIV-specific CD8+ effector cells by canarypox virus. We chose to study individual HVP, who was previously shown to have Gag-specific CD4+ and CD8+ T cells (Table 2). Bulk or CD4-depleted T cells were cocultured with canarypox virus-infected DCs for 7 days. The expansion of Gag-specific CD8+ effector cells was monitored by ELISPOT assay after restimulation with vac-gag-infected monocytes (Fig. 6A) and by cytolytic assay (Fig. 6B). As expected, bulk T cells developed into IFN-γ-secreting and cytotoxic cells. In contrast, CD4-depleted T cells had substantially reduced HIV-specific CD8+ T-cell responses (Fig. 6 A and B, middle). Adding exogenous cytokines in the form of lymphocult to the CD8+ T cells did not restore the HIV-specific expansion observed with the bulk T-cell populations. Taken together, these results suggest that mature DCs infected with can-HIV require the presence of CD4+ T cells to induce strong HIV-specific CD8+ effector responses. The results are representative of three subjects studied.
FIG. 6.
CD4 helper cells are necessary to induce HIV-specific CD8+ T-cell responses. Bulk and CD4-depleted T cells (>98% pure CD8+ T cells by FACS analysis) from individual HVP were cocultured with DCs infected with can-ctl or can-HIV. Exogenous cytokines in the form of lymphocult was added to some of the cocultures of CD8+ T cells and DCs. After 7 days, IFN-γ-producing cells were elicited by restimulation with vaccinia virus-infected monocytes. T-cell cultures from panel A were also tested for cytolytic activity on autologous BLCLs infected with vac-ctl or vac-gag (B) or for p24 reactivity by reexposure to monocytes pulsed with p24 or control protein (C).
Based on the above data, we surmised that can-HIV-infected DCs must expand HIV-specific CD4+ T cells in addition to CD8+ T cells. To test this possibility, bulk or CD4-depleted T cells from the Gag responder HVP were cocultured with DCs infected with either can-ctl or can-HIV for 7 days (Fig. 6C). The T cells were then restimulated for 24 h with autologous monocytes that had been pulsed with either recombinant p24 or control proteins. By using whole protein preparations in the place of poxvirus vectors, we stimulated CD4+ rather than CD8+ T cells. Responding IFN-γ-producing T cells were enumerated by ELISPOT assay. Importantly, p24-specific T cells were detectable only in bulk T-cell populations. As expected, depletion of CD4+ T cells before stimulation with can-HIV-infected DCs abrogated the response to p24 antigen. Addition of cytokines to the CD4-depleted T-cell population restored some, albeit low, p24-specific responses, possibly by expanding the few contaminating CD4+ T cells. Altogether, these results suggest that can-HIV has the capacity to expand antigen-specific CD4+ T cells while it is simultaneously expanding CD8+ T cells, and that this expansion is essential for the development of the CD8 effector response.
Canarypox virus-infected DCs directly stimulate and expand HIV-specific CD4+ T cells.
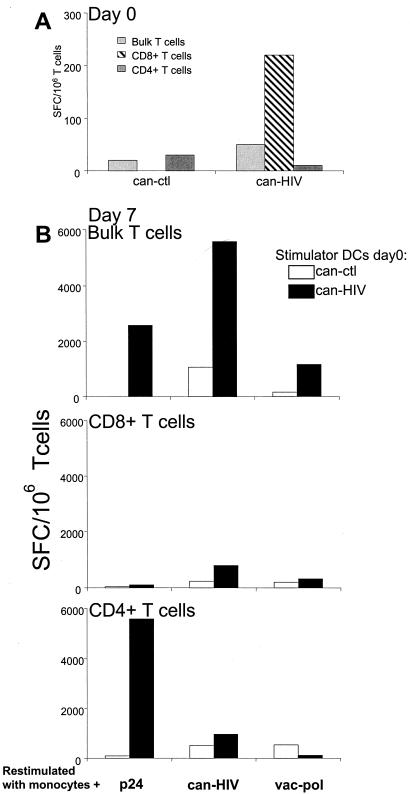
To formally prove that CD4+ T cells could be directly expanded by canarypox virus, we purified CD4+ T cells from selected subjects and cocultured them with can-HIV-infected DCs. Bulk and purified CD8+ T cells were compared alongside. At day 0, we detected significant responses in bulk and purified CD8+ T-cell populations by ELISPOT assay (50 and 220 SFC/106 cells, respectively). In contrast, no significant responses were detected in the CD4+ T-cell population (10 SFC/106 T cells) (Fig. 7A). This is because canarypox virus, like vaccinia virus, induces IFN-γ production primarily from CD8+ T cells in the first 24 h of T cell-APC cocultures (35).
FIG. 7.
DCs infected with canarypox virus expand CD4+ T cells. Bulk, CD4+ and CD8+ T cells from individual VHK were cocultured with DCs that were infected with can-ctl or ctl-HIV. IFN-γ-producing cells were enumerated after 16 h by ELISPOT assay (A) or 7 days after restimulation with monocytes pulsed with p24 protein, control protein, can-ctl or can-HIV (B). HIV-specific values were determined after deducting values for control protein or control vectors.
However, after 7 days of stimulation with can-HIV-infected DCs, high numbers of HIV-specific CD4+ cells could be expanded from both bulk and CD4+ T-cell fractions (2,200 and 5,750 SFC/106 T cells). The expansion was measured by restimulating the T cells with p24-pulsed monocytes in an ELISPOT assay; as expected, p24-pulsed monocytes failed to stimulate IFN-γ production from purified CD8+ T cells (Fig. 7B). Poxvirus-pulsed monocytes (either can-HIV or vac-pol) induced responses only from bulk cultures of T cells, not purified CD4+ and CD8+ T cells. This is consistent with the interpretation that CD8+ T cells require CD4+ T cells to expand and develop into effector cells. Data are representative of three experiments.
In summary, our results confirm that canarypox virus directly stimulates HIV-specific CD4+ T cells in addition to CTL precursors. To our knowledge, this is the first illustration of this vector's ability to stimulate CD4+ helper cell responses. Our results also demonstrate that the activation of antigen-specific CD4+ T cells, unlike that of CD8+ T cells, requires more than 24 h of exposure to canarypox virus-derived antigens.
DISCUSSION
In this study we describe the interaction between DCs and canarypox virus, and we evaluate the ability of canarypox virus-infected DCs to elicit antigen-specific T-cell responses from chronically infected HIV-1+ individuals with known CD8+ T-cell reactivity to HIV-1 antigens. We chose to study mature DCs rather than immature DCs, since they resisted the cytopathic outcome of infection, expressed HIV-1 antigens for sustained periods, and maintained their mature phenotype and function. In all of the HIV-1+ individuals studied here, we successfully elicited CD8+ effector responses using DCs infected with canarypox virus encoding HIV antigens. These reproducible responses consisted of IFN-γ production within 16 h of stimulation, release of antiviral chemokines (RANTES), and/or cytotoxic activity against targets expressing HIV antigens. There was a clear correlation between (i) antigenic specificity between IFN-γ production by vaccinia virus-infected PBMCs and (ii) cytokine secretion and cytolytic activity of T cells stimulated by canarypox-infected DCs. For example, patients HVR, HVP, and HVC showed strong responses to Pol, Gag, and Nef, respectively, in all assays (Table 2; Fig. 2 and 4).
Despite the low frequency of canarypox virus infection, mature DCs presented HIV antigens derived from can-HIV comparably to HLA-restricted peptides and, where tested, even antigens derived from vaccinia virus vectors. This ability of mature DCs to stimulate strong CTL responses with small amounts of foreign protein has been observed with heat-inactivated influenza virus and inactivated Epstein-Barr virus (6, 53). Moreover, when DCs cross-present antigens from apoptotic cells, as few as 1 to 10 apoptotic cells charge 100 DCs efficiently (1). Mature DCs infected with canarypox virus were up to 30 times more potent than monocyte-enriched cells in stimulating anti-HIV CD8+ CTL responses, suggesting that mature DCs are far superior APCs to be targeted in a vaccine formulation. The low levels of stimulatory capacity seen with monocytes may have been dependent on residual DCs in the monocyte preparations. We have found that monocytes, when used at high APC:T ratios, have the capacity to stimulate antigen-specific IFN-γ production from CD8+ T cells in short-term ELISPOT assays using bulk T cells. However, unlike mature DCs, they fail to induce the expansion and full differentiation of CD8+ T cells into cytokine-secreting and cytolytic effector cells (Fig. 3 and reference 36).
HIV-specific cytolytic responses were detected in five of seven individuals studied by this assay. In the remaining two subjects, we were unable to detect specific cytolysis. However, in recall ELISPOT assays, can-HIV-infected DCs readily expanded antigen-specific CD8+ effector cells from these individuals. It is possible that these individuals have HIV-specific CD8+ T cells which lack cytolytic activity secondary to diminished perforin responses (2). As all patients in the cohort were more than 30 years old, they were likely to have been vaccinated against smallpox, and it is known that responses to vaccinia virus are long lived even in HIV-infected individuals (13). In prior canarypox vaccine studies, control vectors were not used at the stimulation level to monitor responses in vitro (5, 11, 17, 19, 21, 22, 48). Our study emphasizes that it is critical to use control vectors to establish the specificity of HIV-specific responses.
An important finding was the requirement for CD4 help to expand antigen-specific CD8+ T cells in response to can-HIV-infected DCs. As canarypox virus induces relatively low protein expression in DCs, there may be insufficient quantities of processed peptide antigens to directly expand CD8+ T cells. CD4 help, in the form of CD40-CD40L interactions which prolong DC viability and induce IL-12 production, would ensure activation of the antiviral CTL response (3, 8, 27, 45, 50, 52). Additional help could come from TRANCE-RANK interactions, which are known to be critical for antiviral responses in animal models (3, 27). The source of help was likely to be HIV-specific CD4+ T cells expanded by canarypox virus-infected DCs, as the addition of nonspecific help in the form of exogenous cytokines failed to restore anti-HIV responses in purified CD8+ T cell populations.
The requirement for antigen-specific CD4+ T cells to expand CD8+ T cells was verified by demonstrating that canarypox virus-infected DCs directly activated p24-specific responses from purified CD4+ T cells. This activation was not evident at early time points in either bulk or CD4+ T-cell populations (i.e., in 24-h ELISPOT assays) but could be detected with 7 days of stimulation. As antigens from canarypox virus are endogenously derived, early on following infection antigens may be more accessible to the MHC class I than the class II pathway. We have previously shown that PBMCs prepared from chronically infected HIV+ individuals produce IFN-γ within 16 to 24 h following infection with canarypox virus, and the response is almost entirely mediated by CD8+ T cells (35). Therefore, rapid effector function induced by canarypox virus (as reflected by IFN-γ production) is likely to be CD4 independent, but the expansion of cytokine-producing and cytotoxic CD8+ T cells, perhaps from true memory T cells, is critically dependent on antigen-specific CD4 helper cells. To our knowledge these experiments provide the first evidence that avipox virus vectors, when targeted to DCs, can simultaneously stimulate and expand antigen-specific CD4+ and CD8+ T cells. CD4+ T cells are critical for the maintenance of antiviral CD8+ T-cell immunity, and data are now emerging to support a correlation between strong helper cell and CD8+ T-cell function in HIV-1-infected individuals (46). Therefore, our observations further validate the use of canarypox virus as a vaccine vector for HIV-1 infection.
Two additional important observations were made in this study. We found that mature DCs could induce IFN-γ-producing CD8+ T cells and recall CTL responses in the absence of repetitive stimulation or cytokines, which are traditionally used to expand HIV-specific CD8+ effector cells in vitro. Prior studies have shown that canarypox virus-infected PBMCs can activate anti-HIV-1 cytolytic effectors (21). However, the canarypox virus activation of CTLs was strictly dependent on cytokines such as IL-2 and IL-7. We also showed that peptide-pulsed DCs were directly able to elicit CTLs that recognized endogenously processed HIV antigens. Mature DCs pulsed with the influenza virus MP peptide can elicit influenza virus CTLs from bulk or purified CD8+ T-cell populations in vitro (36) and can dramatically boost MP-specific effector function when delivered in vivo to healthy volunteers (16). These findings, while consistent with the concept that mature DCs can bypass antigen-specific CD4 help because of increased costimulation and enhanced viability and cytokine production, are harder to reconcile with the requirement for CD4 help by canarypox virus. Pulsing the mature DCs exogenously with a high concentration of peptide may charge sufficient numbers of MHC class I molecules to directly stimulate CD8+ CTL responses. Alternatively, help in the form of nonspecific CD4-DC interactions in our cocultures may have contributed to the development of peptide-specific CD8+ effector cells. Notably, in the absence of MHC class II, DCs are unable to prime CTLs to strong antigen in mice (38). Further studies will be required to determine whether activation of HIV-specific CD8+ T cells by peptide-pulsed DCs requires CD4+ T cells.
Our results using poxviruses and DCs to stimulate CD8+ T-cell responses are in contrast to a previous study in which DCs were unable to stimulate CTL responses from T cells of patients with low CD4 counts (20). One possible explanation for this discrepancy is the use of immature preparations of DCs in that study. Alternatively, since most of the individuals studied here were on therapy and had low to undetectable levels of plasma viremia, CD4 function may have been relatively intact or partially restored. Indeed, many of our patients had demonstrable p24-specific responses by ELISPOT assay. Our results suggest that mature DCs presenting antigen from a canarypox virus vector will successfully expose anti-HIV CTL responses, provided that some HIV-specific CD4+ T cells exist.
Recombinant canarypox viruses administered intramuscularly have an excellent safety profile in humans, but their immunogenicity has been disappointing. Seronegative individuals who have been vaccinated with ALVAC constructs expressing HIV-1 genes demonstrate intermittent responses of variable magnitude (48). This may be because the vectors fail to be acquired by potent APCs such as DCs. Recently we demonstrated that a subcutaneous injection of antigen-pulsed mature DCs elicited in healthy volunteers broad T-cell immunity that was sustained for several months (15). Therefore, mature DCs infected with recombinant canarypox virus vectors expressing HIV genes could constitute effective anti-HIV vaccines, given that they elicit both CD4+ and CD8+ HIV-specific responses. They may be of greatest therapeutic value if delivered to individuals initiated on HAART. While acutely infected HIV-1 patients treated early with HAART can regain HIV-specific CD4+ T-helper responses (46), CD4+ and CD8+ T-cell responses decline with prolonged treatment (41, 44). By targeting canarypox virus vectors to DCs, one could prime or boost immune responses against HIV which involve helper cells, cytolytic responses, and release of antiviral factors.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Judy Adams for graphics and Patrick Haslett for referring individuals to this study.
This work was supported by grants from the Swedish Medical Research Council (K98-99PK-12334-02 [M.L.]) and National Institutes of Health (AI39516 and AI44628 [N.B.]; AI40874 [R.S.]), by a Burroughs Wellcome Fund Clinical Scientist Award (N.B.), and by General Clinical Research Center grant MO1-RR00102 from the National Center for Research Resources at the National Institutes of Health.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–76. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann M F, Wong B R, Josien R, Steinman R M, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bender A, Bui L K, Feldman M A V, Larsson M, Bhardwaj N. Inactivated influenza virus, when presented on dendritic cells, elicits human CD8+ cytolytic T cell responses. J Exp Med. 1995;182:1663–1671. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 8.Bennet S R M, Carbone F R, Karamalis F, Flavell R A, Miller J F. Help for cytotoxic T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 9.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 10.Chun T-W, Stuyver L, Mizell S B, Ehler L A, Mican J A M, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 α and MIP-1 β as the major HIV-suppressive factors produced by CD8- T cells. Science. 1996;270:1811–1816. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 13.Demkowitz W E, Jr, Littaua R A, Wang J, Ennis F A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin J-J, Ait-Yahia S, Patel S, Mattei M-G, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar M, Steinman R M, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe S M, Dunbar P R, Cerundolo V, Nixon D F, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after single injection of mature dendritic cells. J Clin Investig. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhodapkar M V, Krasovsky J, Steinman R M, Bhardwaj N. Mature dendritic cells boost functionally superior T cells in humans without foreign helper epitopes. J Clin Investig. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan M A, Pavlat W A, Tartaglia J, Paoletti E, Weinhold K J, Clements M L, Siliciano R F. Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-range-restricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. J Infect Dis. 1995;171:1623–1627. doi: 10.1093/infdis/171.6.1623. [DOI] [PubMed] [Google Scholar]
- 18.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Schmaljohn A, William C, Steinman R M, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 19.Evans T G, Keefer M C, Weinhold K J, Wolff M, Montefirou D. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp 120 elicits broad and durable CD8+ cytolytic T lymphocyte responses in seronegative volunteers. J Infect Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 20.Fan Z, Huang X L, Zheng L, Wilson C, Borowski L, Liebmann J, Gupta P, Margolick J, Rinaldo C. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J Immunol. 1997;159:4973–4982. [PubMed] [Google Scholar]
- 21.Ferrari G, Berend C, Ottinger J, Dodge R, Barlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 22.Ferrari G, Humphrey W, McElrath M J, Excler J-L, Duliege A-M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K. Latent infection of CD4+ t cells provides a mechanism for lifelong persistance of HIV-1, even in patients on effective combination theraphy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 24.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 25.Furtado M R, Callaway D S, Phair J P, Kunstman K J, Stanton J L, Macken C A, Perelson A S, Wolinsky S M. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 26.Gorse G, Patel G, Mandava M, Belshe R. Vaccine-induced cytotoxic T lymphocytes against human immunodeficiency virus type 1 using two complementary in vitro stimulation stategies. Vaccine. 2000;18:835–849. doi: 10.1016/s0264-410x(99)00323-0. [DOI] [PubMed] [Google Scholar]
- 27.Green E, Flavell R A. TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation. J Exp Med. 1999;189:1017–1020. doi: 10.1084/jem.189.7.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockett R D, Michael Kilby J, Derdeyn C A, Saag M S, Sillers M, Squires K, Chiz S, Nowak M A, Shaw G M, Bucy R P. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med. 1999;189:1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T-cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalams S, Goulder P, Shea A, Jones N, Trocha A, Ogg G, Walker B. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein M R, van Baalen C A, Holweda A M, Kerkhof Garde S R, Bende R J, Keet I R M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopman G, Reutelingsperger C P, Kuijten G A, Keehnen R M, Pals S T, van Oers M H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 34.Koup R A, Safrit J T, Yunzhen C, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoite M A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of pol specific CD8+ T cells in chronically infected HIV-1 positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 36.Larsson M, Messmer D, Somersan S, Fonteneau J-F, Donahoe S M, Lee M, Dunbar B R, Cerundolo V, Julkunen I, Nixon D, Bhardwaj N. Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells. J Immunol. 2000;165:1182–1190. doi: 10.4049/jimmunol.165.3.1182. [DOI] [PubMed] [Google Scholar]
- 37.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone A M, Kuhn M. Dendritic cells need T cell help to prime cytotoxic T cell responses to strong antigens. Eur J Immunol. 1999;29:2826–2834. doi: 10.1002/(SICI)1521-4141(199909)29:09<2826::AID-IMMU2826>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 39.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath J. Cytotoxic-T cell responses viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 40.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1 specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 41.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1998;73:793–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci USA. 1996;93:11354–11358. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrin L, Telenti A. HIV treatment failure: testing for HIV resistance in clinical practice. Science. 1998;280:1871–1873. doi: 10.1126/science.280.5371.1871. [DOI] [PubMed] [Google Scholar]
- 44.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 45.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 47.Rowland-Jones S L, Dong T, Fowke K R, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J, MacDonald K S, McMichael A J. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Investig. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmon-Ceron D, Excler J L, Finkielsztejn L, Autran B, Gluckman J C, Sicard D, Matthews T J, Meignier B, Valentin C, El Habib R, Blondeau C, Raux M, Moog C, Tartaglia J, Chong P, Klein M, Milcamps B, Heshmati F, Plotkin S. Safety and immunogenicity of a live recombinant canarypox virus expressing HIV type 1 gp120 MN MN tm/gag/protease LAI (ALVAC-HIV, vCP205) followed by a p24E-V3 MN synthetic peptide (CLTB-36) administered in healthy volunteers at low risk for HIV infection. AGIS Group and L'Agence Nationale de Recherches sur Le Sida. AIDS Res Hum Retroviruses. 1999;15:633–645. doi: 10.1089/088922299310935. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8(+) lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 50.Schoenberger S P, Toes R E M, van der Voort E I H, Offringa R, Melief C J M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 51.Steinman R M. Dendritic cells. In: Paul W E, editor. Fundamental immunology. 4th ed. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 547–573. [Google Scholar]
- 52.Stuhler G, Zobywalski A, Grunebach F, Bossart P, Reichardt V L, Barth H, Stevanovic S, Brugger W, Kanz L, Schlossman S F. immune regulatory loops determine productive interactions within human T lymphocyte-dendritic cell clusters. Proc Natl Acad Sci USA. 1999;96:1532–1535. doi: 10.1073/pnas.96.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subklewe M, Chahroudi A, Bickham K, Larsson M, Kurilla M G, Bhardwaj N, Steinman R M. Presentation of Epstein-Barr virus latency antigens to CD8+, interferon-γ-secreting, T lymphocytes. Eur J Immunol. 1999;29:3995–4001. doi: 10.1002/(SICI)1521-4141(199912)29:12<3995::AID-IMMU3995>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Tartaglia J, Jarrett O, Neil J C, Desmettre P, Paoletti E. Protection of cats against feline leukemia virus by vaccination with a canarypox virus recombinant, ALVAC-FL. J Virol. 1993;67:2370–2375. doi: 10.1128/jvi.67.4.2370-2375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- 56.Vermes I, Haanen C, Steffens-Nakken H, Reuteligsperger C. A novel assay for apoptosis—flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 57.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. β-Chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 58.Wasik T, Wierzbicki A, Whiteman V, Trinchieri G, Lischner H, Kozbor D. Association between HIV-specific T helper responses and CTL activities in pediatric AIDS. Eur J Immunol. 2000;30:117–127. doi: 10.1002/1521-4141(200001)30:1<117::AID-IMMU117>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 60.Yang O O, Kalams S A, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker B D, Johnson R P. Efficient lysis of human immunodeficiency virus type-1 infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 62.Zhou L-J, Tedder T F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]