Abstract
Help for the induction of cytolytic T lymphocytes is mediated by dendritic cells (DC) that are conditioned by CD40 signaling. We identified tumor necrosis factor family member CD27L/CD70, which is expressed by cytolytic T lymphocytes on interaction with DC to control CD154 (CD40L) up-regulation on CD45RA+ helper T cells for subsequent DC stimulation. The results show that the initiation of a cytolytic immune response is determined by regulatory circuits, requiring simultaneous activation and differentiation of all cells involved in T lymphocyte–DC cluster formation.
On activation in the periphery, early dendritic cells (DC) differentiate and move antigen into draining lymph nodes. During this process, DC lose their capacity to capture antigen. However, after maturation, DC up-regulate plasma-membrane class II molecules loaded with antigenic fragments, forming stable peptide/major histocompatibility complexes (MHC) capable of stimulating T cells (1–3). In T cell-rich areas of lymph nodes, DC rapidly cluster with an abundant number of T lymphocytes to initiate or to anergize specific T cell responses (4).
DC excel at processing and presenting exogenous viral, bacterial, tumor, and transplantation antigens in association with class I and class II MHC molecules to cytolytic T lymphocytes (CTL) and helper T cells (Th; ref. 5), and DC form long lasting and high avidity conjugates with T cells that are specific for the antigen presented (6, 7). Thus, the microenvironment for the primary induction of a cytolytic immune response is a multicell complex in which distinct cell types are in close proximity, enabling signaling by surface molecules and short-range lymphokines. Recent reports indicate that Th are required for cross-priming and efficient induction of specific CTL responses (8). In this respect, the DC is the entity that links and coordinates antigen-specific activation events by presenting epitopes for both Th and CTL (4, 9).
In spite of its importance, regulatory elements that initiate productive interactions within T lymphocyte–DC clusters are not defined well. Tumor necrosis factor family members CD27L/CD70 and CD40L/CD154 are rapidly inducible molecules preferentially expressed by lymphocytes. Although CD70 is expressed on activated, but not on resting, T cells, its ligand CD27 is expressed stably on virtually all CD45RA+ and, at lower levels, on the majority of CD45RO+ type T cells (10, 11). Disruption of CD27L/CD70 signaling in mixed lymphocyte reactions as well as in phytohemagglutinin-, anti-CD2-, or anti-CD3-induced T cell stimulations results in abrogation of cytolytic or proliferative responses, thus underscoring the importance of this costimulatory pathway for T cell activation and T–T cell interactions (12). Moreover, recently CD27L/CD70 costimulation has been estimated as equal if not superior to CD80/CD86 signaling in a mouse-tumor model in vivo (13). CD40L/CD154 is expressed preferentially on activated, but not on resting, Th, and CD40 is expressed highly on professional antigen-presenting cells (APC), suggesting important regulatory functions in T cell–DC interactions (14). Importantly, the crucial role of CD40L/CD154 ligation for DC maturation and differentiation has been reported in various model systems, and DC, stimulated by CD154-expressing Th, are potent activators of murine CTL in vitro as well as in vivo (15–17).
Here, we show that human CTL, on interaction with DC, up-regulate CD70 molecules for subsequent stimulation of CD45RA+ Th. CD70-costimulated Th cells up-regulate interleukin (IL) 2 and interferon (IFN) γ for mutual interaction with CTL and CD154 molecules to condition DC for efficient CTL induction.
MATERIALS AND METHODS
Cells.
Cells were prepared by using previously described methods and reagents (18). Briefly, HLA-A2-positive human peripheral blood mononuclear cells were isolated from healthy individuals by Ficoll density gradient centrifugation (Amersham Pharmacia). T lymphocyte subsets were negatively selected by using mAbs reactive with CD3, CD4, CD8, CD14, CD45RO, CD45RA, MHC class II, κ, λ, and goat-anti-mouse IgG-conjugated magnetic beads (PerSeptive Diagnostics, Cambridge, MA) or Multisort Macs Reagents (Miltenyi Biotec, Auburn, CA). CTL and Th thus obtained were >95% CD8 and CD4 positive, respectively, as determined by flow cytometry analysis. CD4/CD45RA and CD4/CD45RO populations were >90% positive for CD45RA or CD45RO, and monocytes were >85% reactive with anti-CD14 mAb, containing less than 3% contaminating B or T lymphocytes.
DC were generated by culturing monocytes in the presence of 100 ng/ml granulocyte–macrophage colony-stimulating factor (Leukomax, Novartis, Basel, Switzerland) and 1,000 units/ml IL-4 (Genzyme) for 7 days, according to published protocols (19). DC thus obtained show clear dendritic morphology and high expression of MHC class I and class II, as well as CD40 and CD54, but were negative for CD14 molecules. In some experiments, metrizamide-enriched DC were isolated according to manufacturer’s instructions (Sigma).
The murine pre-B cell line 300-19 was transfected with cDNA encoding human CD27 or CD70 molecules or with the vector alone (mock) as described (18). The transfectants were fixed with 2% neutral-buffered paraformaldehyde before they were added to the cultures.
Antigens/Peptides.
The HIV-RT476–484 (ILKEPVHGV; ref. 20) and the GP2 (IISAVVGIL; ref. 21) peptides were prepared according to published sequences with an automated peptide synthesizer (Applied Biosystems model 432A) using fluorenylmethoxycarbonyl chemistry and analyzed by HPLC. These peptides carrying HLA-A2 motifs were used as epitopes for CTL. The Pan-DR binding peptide [PADRE; a(X)VAAWTLKAAa; ref. 22] was used as helper epitope.
In Vitro Cultures and Assays.
Cultures were set up in α-MEM (GIBCO/BRL) supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), 10% mercaptoethanol, and 10% heat-inactivated fetal calf serum. DC were pulsed for 2 h with 20 μg/ml of the indicated peptide antigens before being added to the cultures. Cells of the indicated types and numbers were mixed in 200-μl cultures in 96-well flat-bottom plates and incubated for the indicated times, after which 51Cr-release assays, flow cytometry analysis, and ELISA techniques were performed.
Specific lysis was determined by adding 5 × 103 51Cr-labeled, HLA-A2 positive, the antigen processing mutant lymphoblast hybrid T2 (147xCEM.T2) targets to the cultured cells. T2 cells were pulsed for 2 h with 20 μg/ml HIV-RT or GP2 peptides before 51Cr-labeling. Spontaneous release and maximum release was determined by incubation of 51Cr-labeled T2 cells in medium or detergents, respectively. The percentage of specific lysis was assessed after a 4- to 6-h incubation at 37°C as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Standard deviations of duplicates were <15% of the specific lysis values shown.
Lymphokine analyses for IL-2, IL-4, IFN-γ, and IL-12 were carried out by using ELISA techniques for secreted cytokines, according to manufacturer’s instructions (Becton Dickinson for IL-2, IL-4, and IFN-γ; Genzyme for IL-12).
CD27, CD70, and CD154 expression was determined by three-color flow cytometry analysis. T lymphocytes (5 × 105) of the indicated type were cultured as duplicates with DC (105) in the presence of CD27, CD70, and mock transfectants overnight in 96-well plates. T cells were stimulated by using superantigens (SAGs; 0.1 ng/ml staphylococcal enterotoxin A, 0.1 ng/ml staphyloenterotoxin B, and 0.1 ng/ml toxic shock syndrome toxin 1; Sigma) for the indicated times and analyzed with chromophore-labeled antibody (Ab) reactive with CD27 (phycoerythrin), CD70 (fluorescein isothiocyanate), CD154 (fluorescein isothiocyanate), CD45RA (phycoerythrin), and CD3 (APC) on a FacsCalibur cytometer (Becton Dickinson). For detection of CD154, 2 μg/200 μl anti-CD40 Ab (Alexis, Grunberg, Germany) were added to the cultures during SAG stimulation.
RESULTS AND DISCUSSION
CD70 Positively Regulates Induction of Specific Cytotoxicity Within T Lymphocyte–DC Clusters.
Immune regulatory mechanisms were studied by using a modified system for primary induction of human-peptide-specific CTL in vitro. HLA-A2 positive DC were used to present HIV-RT-derived peptides and PADRE to autologous CTL and helper T lymphocytes, respectively. Cluster formation of DC and, typically, 5–20 T lymphocytes occurred within 1 day of starting the cultures, when DC changed from a nonadherent phenotype to a phenotype that was adherent and had elongated cells (9). The induction of peptide-specific cytotoxicity was assessed after 9 days by the ability of the cultured cells to lyse peptide-pulsed T2 target cells in a standard 51Cr-release assay.
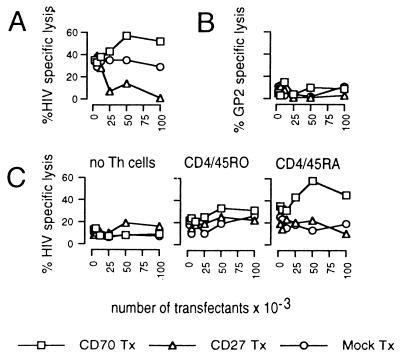
To determine the significance of CD27L/CD70 interaction within human T lymphocyte–DC clusters, we analyzed the effects of murine 300-19 cells transfected with cDNA coding for human CD27 [CD27 transfectant (Tx)], CD70 (CD70 Tx), or the empty vector (mock Tx) on the efficiency of CTL induction. The data presented in Fig. 1A show increased lysis of HIV-RT-coated T2 target cells after the addition of CD70 Tx to cultures containing Th, CTL, and DC, compared with mock controls. Conversely, the addition of CD27 Tx to these cultures obviously inhibited lysis of T2 targets presenting HIV-RT epitopes, thus clearly indicating the essential role of CD27L/CD70 ligation in the regulatory process of CTL induction. T2-target cells expressing the irrelevant HER2/neu-derived peptide GP2 (21) were not lysed in HIV–PADRE-stimulated cell cultures, thus confirming the specificity of the cells induced (Fig. 1B). No lysis of HIV-RT-pulsed T2 cells was observed in cases where either DC or CTL were omitted (not shown). Moreover, addition of the transfectants to HIV–PADRE-stimulated T lymphocytes during the 51Cr-release assay had no effect on the lysis of target cells expressing the appropriate antigen, suggesting that CD27L/CD70 signaling is crucial for induction of CTL and does not interfere with effector functions (not shown).
Figure 1.
CD27L/CD70 signaling is essential for CTL induction. T lymphocytes (106) were incubated with various numbers of paraformaldehyde-fixed CD70 Tx, CD27 Tx, or mock Tx and autologous DC (104) presenting HIV-RT peptides for CTL and PADRE helper epitopes. After 9 days, induction of specific cytolytic activity was determined by the ability of cultured cells to lyse 5,000 51Cr-labeled T2 target cells coated with HIV-RT epitopes (A) or irrelevant GP2 peptides (B). (C) Metrizamide-enriched DC (3 × 104) were pulsed with HIV and PADRE peptides and cultured with CTL (5 × 105) and CD45RO+ Th (5 × 105), CD45RA+ Th (5 × 105), or without Th cells in the presence of various numbers of fixed CD27 Tx, CD70 Tx, or mock Tx. After 9 days, specific lysis of HIV peptide-coated T2 cells was determined as described. Specific lysis of T2-targets expressing an irrelevant antigen was <15% in this culture. Data are representative for five independent assays. Standard deviations were <15% of the values shown.
Previous reports indicate that CD27, the ligand for CD70, is expressed differentially and regulated on Th subsets defined by their CD45 isomorphs. With this information, we next set out to define T cell populations that are affected by CD27L/CD70 signaling. Consequently, we separated CD45RA+ and CD45RO+ Th to culture with purified CD8+ CTL, metrizamide-enriched DC presenting HIV and PADRE-peptides and CD27 Tx, CD70 Tx, or mock Tx, respectively. When this regime is used, CD70, but not CD27 or mock-transfected cells, significantly enhanced lysis of HIV peptide-coated T2 targets in cultures where CD45RA+ Th were recruited for CTL induction (Fig. 1C). In contrast, no overt activation of peptide-specific cytotoxicity was observed in cases in which Th were omitted or in situations where CD45RO+ Th cells were used for CTL induction. The data clearly indicate the essential role for CD45RA+ Th cells in CTL induction, and this requirement is not overcome by the addition of CD70 Tx. To exclude the possibility that the distinct Th subsets may respond differentially to the helper epitope used in this system, we performed proliferation assays using DC to present PADRE peptides to separated CD45RA+ or CD45RO+ Th cells. No significant difference in the proliferative capacity was found, suggesting that both Th subsets recognize and are activated by PADRE peptides (not shown).
CTL, but Not Th, Rapidly Up-Regulate CD70 Molecules on Activation.
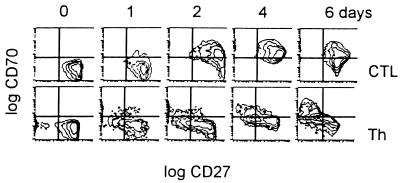
We next set out to identify the cell population physiologically expressing CD70 antigens within T lymphocyte–DC clusters. Negatively separated Th and cytotoxic T cell subsets were stimulated with SAGs in the presence of autologous DC. Expression of CD70 and CD27 antigens was monitored over a period of 6 days. SAG were used to provide a means of polyclonal T cell activation under conditions mimicking best T cell antigen receptor/MHC/peptide affinities in the presence of DC costimulation (23, 24). Consistent with previously published data, we found that CD8+ CTL uniformly and rapidly up-regulate CD70 molecules, with peak expression between day 2 and 4 as assessed by three-color flow cytometry analysis (Fig. 2; ref. 25). In contrast, CD4+ Th did not express a significant amount of CD70 molecules until 6 days after stimulation, when two distinct subpopulations emerged mutually exclusively expressing CD70 and CD27 antigens. The significance of these findings was confirmed by using different activation procedures. CTL that were stimulated with soluble OKT-3 Ab in the presence of DC displayed CD70 expression comparable to the data shown. On the other hand, OKT-3 stimulated Th in the absence of DC and converted into an activated phenotype but did not express CD70 molecules exceeding >10% positive cells within the 6 days of the experiment (not shown). Interestingly, by using a phorbol 12-myristate 13-acetate/ionomycin regime without DC, 40% of both CTL and Th cells stained positive for CD70 molecules, suggesting that both T cell subsets are indeed capable of CD70 up-regulation (not shown). In the mouse system, it has been shown that the presence of MHC class II molecules with APC stimulate CD45RBhi “naive” type Th subset and negatively regulate the CD45RBlo “memory” type Th subset that is recognized to express CD70 antigens on activation. Because the regimes using DC for Th stimulation resulted in low CD70 expression, it is possible that a similar mechanism may be responsible for the effects we observed (26).
Figure 2.
CTL, but not Th cells, rapidly up-regulate CD70 on activation. Negatively selected CD8 and CD4 cells (5 × 105) were stimulated with SAG (0.1 ng/ml staphylococcal enterotoxin A, 0.1 ng/ml staphylococcal enterotoxin B, and 0.1 ng/ml toxic shock syndrome toxin 1) in the presence of autologous DC (105). After the indicated times, three-color flow cytometry analysis was performed by using fluorochrome-conjugated Ab with specificities for CD70 (fluorescein isothiocyanate), CD27 (phycoerythrin), and CD3 (APC). Gates were set on CD3+ lymphocytes. Data are representative for two independent assays.
CD70 Molecules Costimulate CD45RA Th Lymphocytes to Produce IL-2 and IFN-γ.
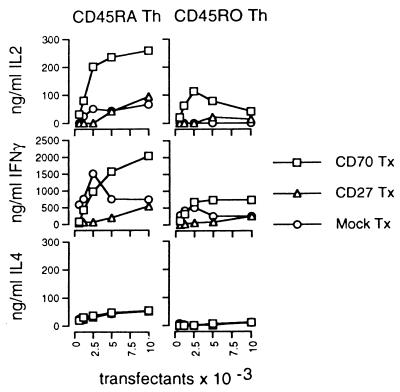
With this information, we studied the effects of CD70-expressing CTL on Th subsets within the clusters. CD70 Tx, CD27 Tx, and mock Tx were used to mimic and control the influence of activated CTL on Th induction. DC presenting PADRE helper epitopes were used to stimulate isolated CD45RA+ or CD45RO+ Th in the presence of the transfectants. IL-2 contents in the supernatants were analyzed after 1 day, and IL-4 and IFN-γ concentrations were assessed after 4 days of culture by using ELISA techniques. As shown in Fig. 3, CD70 Tx greatly stimulate CD45RA Th, but not CD45RO Th, to produce IL-2 and IFN-γ; this stimulation was much greater than that of mock controls. However, in these cultures, no significant difference was found in the ability of the transfectants to stimulate the Th subsets to produce IL-4. The results shown strongly support the hypothesis that CD70-expressing CTL may provide costimulatory support for CD45RA Th activation via CD70–CD27 interaction. Interestingly, APC-derived signals like CD80, CD86, or IL-12, as well as Th-derived IL-2, have been reported to mediate CD70 up-regulation, suggesting that the expression of IL-2 and CD70 molecules is positively regulated mutually (27).
Figure 3.
CD70 costimulates CD45RA+ Th for IL-2 and IFN-γ production. CD45RA+ or CD45RO+ Th (5 × 105) were cultured in duplicates with autologous DC (104) presenting PADRE helper epitopes and varying numbers of CD27, CD70, and mock Tx. IL-2 concentrations were determined after 1 day, and IL-4 and IFN-γ concentrations were assessed after 4 days in the supernatants by using ELISA techniques. Standard deviations were <15% of the values shown.
CD70 Molecules Induce CD45RA+ Th to Up-Regulate CD154 Antigens.
Recently, it has been shown that help for CTL generation is mediated by DC that are conditioned by CD154+ Th (15–17). Importantly, CD154 molecules are expressed preferentially on CD45RA+ helper T lymphocytes (28) and are recognized to provide crucial stimulatory signals for the differentiation of DC leading to IL-12 production and up-regulation of CD80, CD86, and other molecules for T cell activation (29).
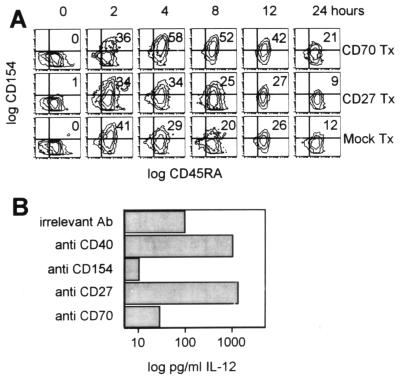
To test whether CD70-expressing CTL may enhance CD154 up-regulation on Th for subsequent DC stimulation, we cultured CD45RA+ Th and DC in the presence of CD27 Tx, CD70 Tx, or mock Tx. CD154 expression was monitored after the addition of SAG to the cultures by using three-color flow cytometry. As shown in Fig. 4A, the addition of CD70 Tx to these cultures resulted in enhanced and prolonged expression of CD154 by CD45RA+ Th, compared with mock controls. However, no overt stimulation was seen in cultures set up with CD27 Tx.
Figure 4.
CD70 determines CD154 expression by CD45RA+ Th for DC stimulation. (A) CD45RA+ helper T lymphocytes (5 × 105) were cocultured overnight with autologous DC (105) and fixed CD70 Tx, CD27 Tx, or mock Tx (5 × 104). T cells were stimulated with SAG (0.1 ng/ml staphylococcal enterotoxin A, 0.1 ng/ml staphylococcal enterotoxin B, and 0.1 ng/ml toxic shock syndrome toxin 1) for the indicated time, stained with CD154 (fluorescein isothiocyanate), CD45RA (phycoerythrin), and CD3 (APC)-conjugated mAb, and analyzed by flow cytometry. Cells were gated on the CD3+ population. (B) CD45RA+ Th cells (106), DC presenting PADRE epitopes (105), and CD70 Tx (105) were cocultured with the indicated Ab. After 36 h, IL-12 concentrations in the supernatants were determined by using ELISA techniques. Results shown are representive for three independent assays.
To demonstrate further that CD70-costimulated Th directly induce DC maturation, we cocultured CD45RA+ Th cells, CD70 Tx, and DC expressing PADRE epitopes in the presence of various Ab. After 36 h, concentrations of DC-derived IL-12 in the supernatants were assayed with ELISA techniques. As shown in Fig. 4B, cross-linking of CD40 molecules on DC resulted in significant IL-12 production that is blocked by Ab directed against CD154 molecules expressed by CD45RA+ Th cells. Most importantly, CD45RA+ Th cells, if activated by the addition of CD27-specific Ab-stimulated DC, produce more IL-12 than CD40 cross-linking. In contrast, Ab directed against CD70 molecules that were expressed exclusively on the transfectants effectively inhibited productive Th–DC interactions, leading to reduced IL-12 concentrations in the supernatants. These findings thus provide striking evidence that CD70-expressing CTL recruit Th not only for mutual interaction but to condition them for efficient DC stimulation.
CONCLUSIONS
Recently, a dynamic model of Th-dependent CTL induction was suggested in which DC first get activated by CD154+ Th to stimulate the binding of CTL (17). Here, we extend this model by showing that within DC–T cell clusters, Th, CTL, and DC mutually control each other via regulatory loops.
The data presented here support the view of a multistep activation process of cytolytic immune responses. By up-regulating CD70 molecules, antigen-activated CTL costimulate CD45RA+ helper T lymphocytes to fulfill two tasks: (i) they back-stimulate CTL by producing IL-2 and IFN-γ, and (ii) by up-regulating CD154 molecules, Th signal DC to differentiate and produce IL-12, thus facilitating specific CTL induction.
This hypothesis is in agreement with the two-signal model as originally postulated without a need for a “first effector Th cell” to initiate productive collaboration. CTL may efficiently recruit and costimulate Th that recognize their respective antigens on the DC within the same cell cluster (30). In this context, control of Th activation by specific CTL may greatly reduce unspecific bystander activation and the risk of unnecessary cytokine release, factors that are indeed pivotal for primary CTL induction in many systems. This model does not rule out that Th that have been activated in the controlled setting of the clusters may interact serially and activate other DC presenting processed antigen. The model predicts, however, that the initiation of cytolytic immune responses is controlled by mutual activation within clusters, implying parallel differentiation and maturation processes of T lymphocytes and DC.
ABBREVIATIONS
- Ab
antibody
- APC
antigen-presenting cells
- CTL
cytolytic T lymphocytes
- DC
dendritic cells
- IFN
interferon
- IL
interleukin
- MHC
major histocompatibility complex
- PADRE
Pan-DR binding peptide
- SAG
superantigen
- Th
helper T cells
- Tx
transfectant
References
- 1.Sousa C R, Hieny S, Scharton K T, Jankovic D, Charest H, Germain R N, Sher A. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature (London) 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature (London) 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M C, Mitchison N A, Schneider S C. Folia Biol (Prague) 1994;40:359–369. [PubMed] [Google Scholar]
- 5.Albert M L, Sauter B, Bhardwaj N. Nature (London) 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 6.Inaba K, Steinman R M. Science. 1985;229:475–479. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi G, Karjalainen K, Lanzavecchia A. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 8.Ossendorp F, Mengede E, Camps M, Filius R, Melief C J. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuhler G, Schlossman S F. Proc Natl Acad Sci USA. 1997;94:622–627. doi: 10.1073/pnas.94.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hintzen R Q, de Jong R, Lens S M, Brouwer M, Baars P, van Lier R A. J Immunol. 1993;151:2426–2435. [PubMed] [Google Scholar]
- 11.Agematsu K, Kobata T, Sugita K, Freeman G J, Beckmann M P, Schlossman S F, Morimoto C. J Immunol. 1994;153:1421–1429. [PubMed] [Google Scholar]
- 12.Sugita K, Torimoto Y, Nojima Y, Daley J F, Schlossman S F, Morimoto C. J Immunol. 1991;147:1477–1483. [PubMed] [Google Scholar]
- 13.Nieland J D, Graus Y F, Dortmans Y E, Kremers B L, Kruisbeek A M. J Immunother. 1998;21:225–236. doi: 10.1097/00002371-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Vanbervliet B, Dubois B, van Kooten C, Durand I, Banchereau J. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett S, Carbone F R, Karamalis F, Flavell R A, Miller J, Heath W R. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberger S, Toes R, van der Voort E, Offringa R, Melief C. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 17.Ridge J, DiRosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 18.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman S F, Morimoto C. Proc Natl Acad Sci USA. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsomides T, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peoples G, Smith R C, Linehan D C, Yoshino I, Goedegebuure P S, Eberlein T J. Cell Immunol. 1995;164:279–286. doi: 10.1006/cimm.1995.1171. [DOI] [PubMed] [Google Scholar]
- 22.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra H M, Kubo R T, Sette A, et al. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 23.Leder L, Llera A, Lavoie P M, Lebedeva M I, Li H, Sekaly R P, Bohach G A, Gahr P J, Schlievert P M, Karjalainen K, et al. J Exp Med. 1998;187:823–833. doi: 10.1084/jem.187.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haffner A C, Zepter K, Elmets C A. Proc Natl Acad Sci USA. 1996;93:3037–3042. doi: 10.1073/pnas.93.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brugnoni D, Airo P, Marino R, Notarangelo L D, van Lier R A, Cattaneo R. Immunol Lett. 1997;55:99–104. doi: 10.1016/s0165-2478(96)02693-4. [DOI] [PubMed] [Google Scholar]
- 26.Farber D L, Luqman M, Acuto O, Bottomly K. Immunity. 1995;2:249–259. doi: 10.1016/1074-7613(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 27.Lens S M, Baars P A, Hooibrink B, van Oers M H, van Lier R A. Immunology. 1997;90:38–45. doi: 10.1046/j.1365-2567.1997.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludewig B, Henn V, Schroder J M, Graf D, Kroczek R A. Eur J Immunol. 1996;26:3137–3143. doi: 10.1002/eji.1830261246. [DOI] [PubMed] [Google Scholar]
- 29.Cella M, Scheidegger D, Palmer L K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bretscher P. Immunol Today. 1992;13:74–76. doi: 10.1016/0167-5699(92)90138-W. [DOI] [PubMed] [Google Scholar]