Abstract
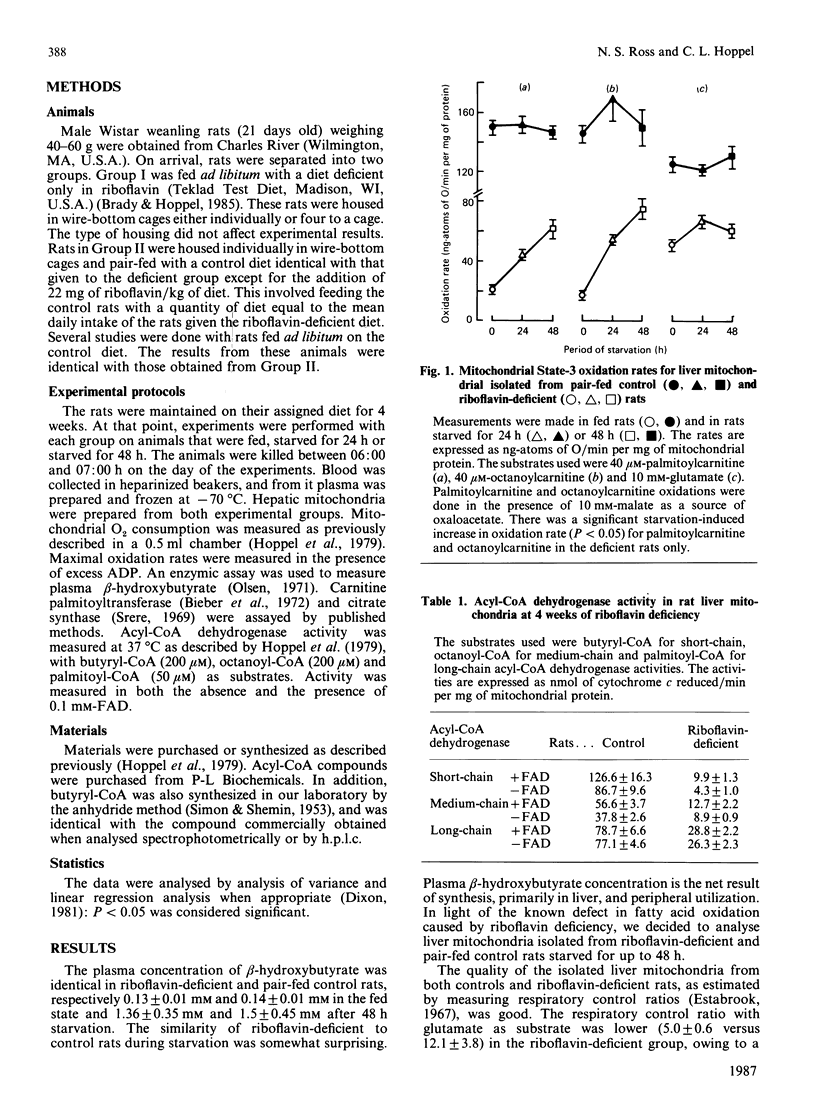
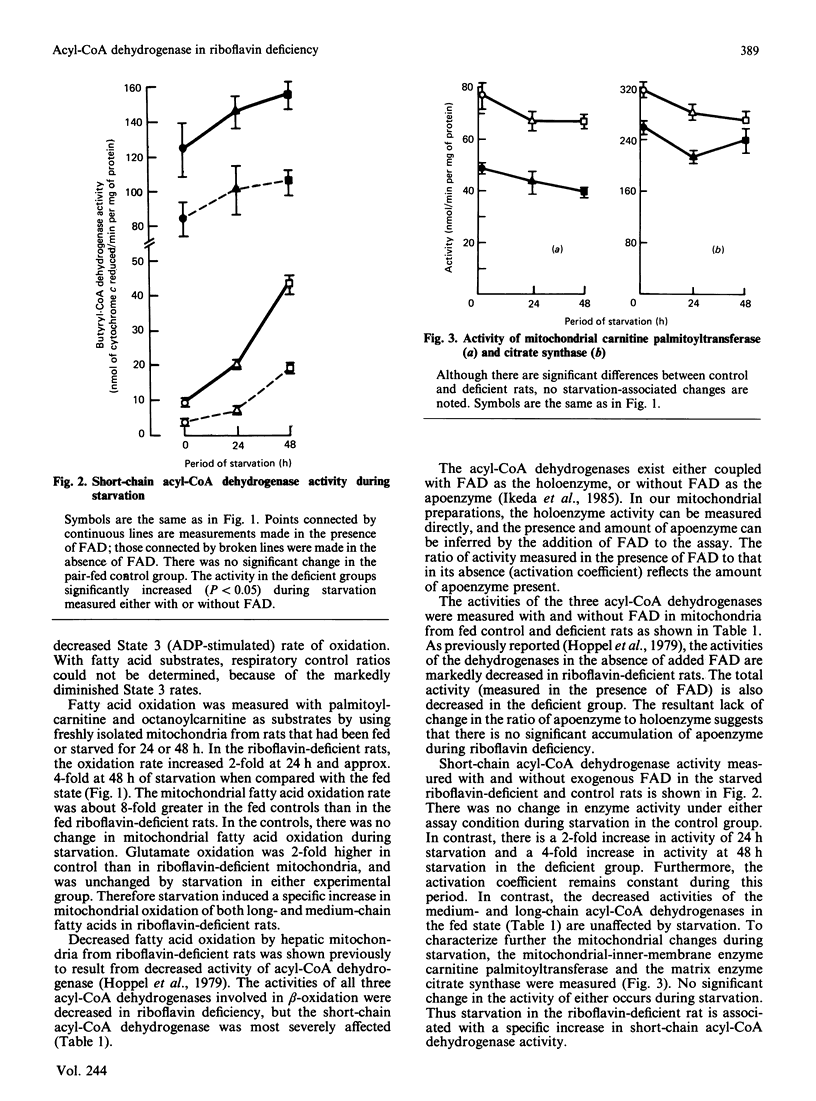
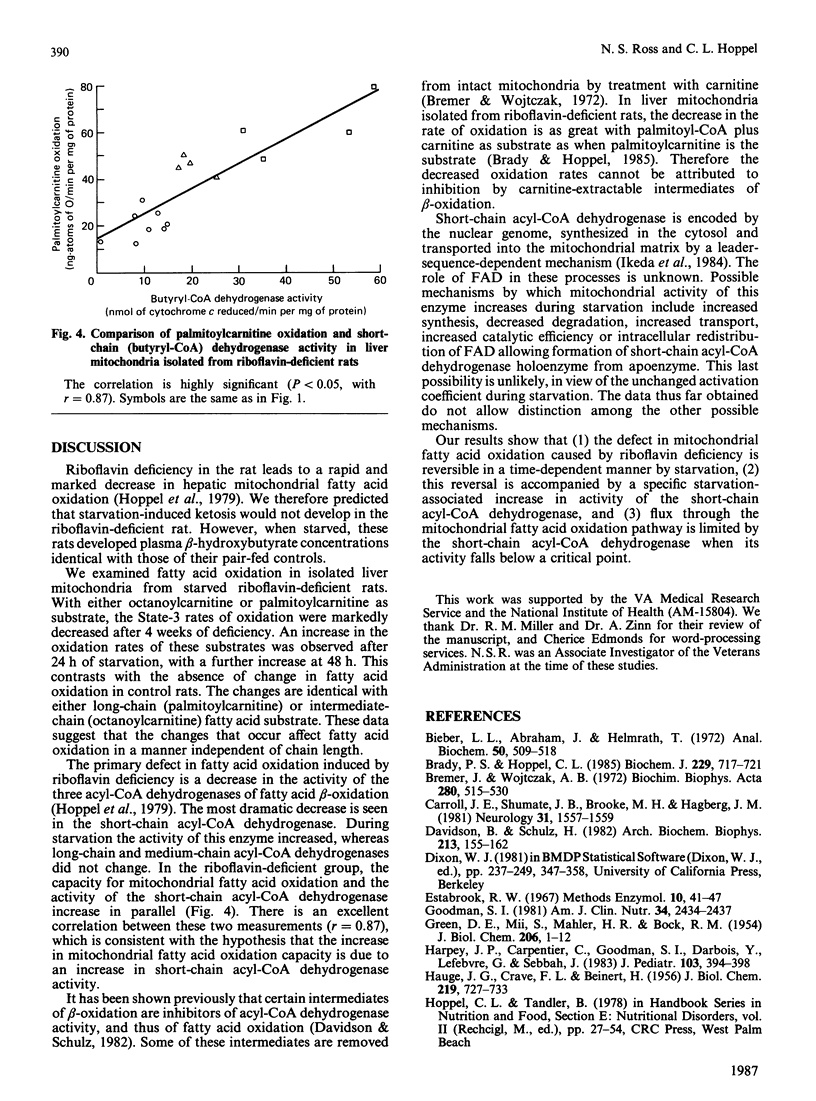
Riboflavin deficiency in weanling rats causes a metabolic disorder characterized by failure to oxidize fatty acids. The disorder is similar to that seen in several human diseases, some of which are responsive to pharmacological doses of riboflavin. Previous analysis of the riboflavin-deficient rat has shown that the failure of fatty acid oxidation is due to a decrease in the activity of the acyl-CoA dehydrogenases of beta-oxidation. The activity of these flavoenzymes in liver rapidly decreases when a riboflavin-deficient diet is initiated. The objectives of these experiments were to analyse the effects of starvation on liver mitochondria isolated from the riboflavin-deficient rat. Our studies show that the decreased mitochondrial fatty acid oxidation induced by riboflavin deficiency is partially reversed by starvation. The extent of this reversal is proportional to the duration of starvation. The starvation-associated increase in fatty acid oxidation is mediated by an increase in the mitochondrial short-chain acyl-CoA dehydrogenase activity. The activity of this enzyme is increased such that the ratio of short-chain acyl-CoA dehydrogenase apoenzyme to holoenzyme does not change. We conclude that short-chain acyl-CoA dehydrogenase activity is limiting for fatty acid oxidation when its activity falls below a critical point. The increased mitochondrial specific activity of short-chain acyl-CoA dehydrogenase during starvation may result from an increased availability of flavin coenzyme or an increase in enzyme catalytic efficiency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L., Abraham T., Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972 Dec;50(2):509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Brady P. S., Hoppel C. L. Hepatic peroxisomal and mitochondrial fatty acid oxidation in the riboflavin-deficient rat. Biochem J. 1985 Aug 1;229(3):717–721. doi: 10.1042/bj2290717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Carroll J. E., Shumate J. B., Brooke M. H., Hagberg J. M. Riboflavin-responsive lipid myopathy and carnitine deficiency. Neurology. 1981 Dec;31(12):1557–1559. doi: 10.1212/wnl.31.12.1557. [DOI] [PubMed] [Google Scholar]
- Davidson B., Schulz H. Separation, properties, and regulation of acyl coenzyme A dehydrogenases from bovine heat and liver. Arch Biochem Biophys. 1982 Jan;213(1):155–162. doi: 10.1016/0003-9861(82)90450-7. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., MII S., MAHLER H. R., BOCK R. M. Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):1–12. [PubMed] [Google Scholar]
- Goodman S. I. Organic aciduria in the riboflavin-deficient rat. Am J Clin Nutr. 1981 Nov;34(11):2434–2437. doi: 10.1093/ajcn/34.11.2434. [DOI] [PubMed] [Google Scholar]
- HAUGE J. G., CRANE F. L., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. III. Palmityl coA dehydrogenase. J Biol Chem. 1956 Apr;219(2):727–733. [PubMed] [Google Scholar]
- Harpey J. P., Charpentier C., Goodman S. I., Darbois Y., Lefèbvre G., Sebbah J. Multiple acyl-CoA dehydrogenase deficiency occurring in pregnancy and caused by a defect in riboflavin metabolism in the mother. Study of a kindred with seven deaths in infancy: Value of riboflavin therapy in preventing this syndrome. J Pediatr. 1983 Sep;103(3):394–398. doi: 10.1016/s0022-3476(83)80410-7. [DOI] [PubMed] [Google Scholar]
- Hoppel C., DiMarco J. P., Tandler B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem. 1979 May 25;254(10):4164–4170. [PubMed] [Google Scholar]
- Olsen C. An enzymatic fluorimetric micromethod for the determination of acetoacetate, -hydroxybutyrate, pyruvate and lactate. Clin Chim Acta. 1971 Jul;33(2):293–300. doi: 10.1016/0009-8981(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Stanley C. A., Hale D. E., Coates P. M., Hall C. L., Corkey B. E., Yang W., Kelley R. I., Gonzales E. L., Williamson J. R., Baker L. Medium-chain acyl-CoA dehydrogenase deficiency in children with non-ketotic hypoglycemia and low carnitine levels. Pediatr Res. 1983 Nov;17(11):877–884. doi: 10.1203/00006450-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Bartlett K., Stevens D. L., Alberti K. G., Gibson G. J., Johnson M. A., McCulloch A. J., Sherratt H. S. Short-chain acyl-CoA dehydrogenase deficiency associated with a lipid-storage myopathy and secondary carnitine deficiency. N Engl J Med. 1984 Nov 8;311(19):1232–1236. doi: 10.1056/NEJM198411083111906. [DOI] [PubMed] [Google Scholar]


