Abstract
An equation is derived from first principles for describing the change in concentration with time of a beta-lactam antibiotic in the presence of intact Gram-negative bacteria possessing a beta-lactamase located in the periplasmic space. The equation predicts a first-order decline in beta-lactam concentration of the form [S] = [Si]e lambda t, where [S] is the exogenous concentration of beta-lactam, [Si] is the value of [S] at time zero, t is the time from mixing of cells and antibiotic and lambda (less than 0) is the decay constant. The value of lambda is exactly described by the theory in terms of experimentally measurable quantities. Quantitative data concerning cephaloridine hydrolysis by intact cells of Haemophilus influenzae agreed well with the theory, as did data concerning the uptake of 2-nitrophenyl galactoside by intact cells of Escherichia coli. Cephalosporin C hydrolysis by intact cells of Pseudomonas aeruginosa did not progress as predicted by the theory. The theory is applicable to any substrate which is acted on by an enzyme that is located solely in the periplasmic space and that obeys the Michaelis-Menten equation of enzyme kinetics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Dorrance D. The permeability barrier of Haemophilus influenzae type b against beta-lactam antibiotics. J Antimicrob Chemother. 1983 Nov;12(5):435–449. doi: 10.1093/jac/12.5.435. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
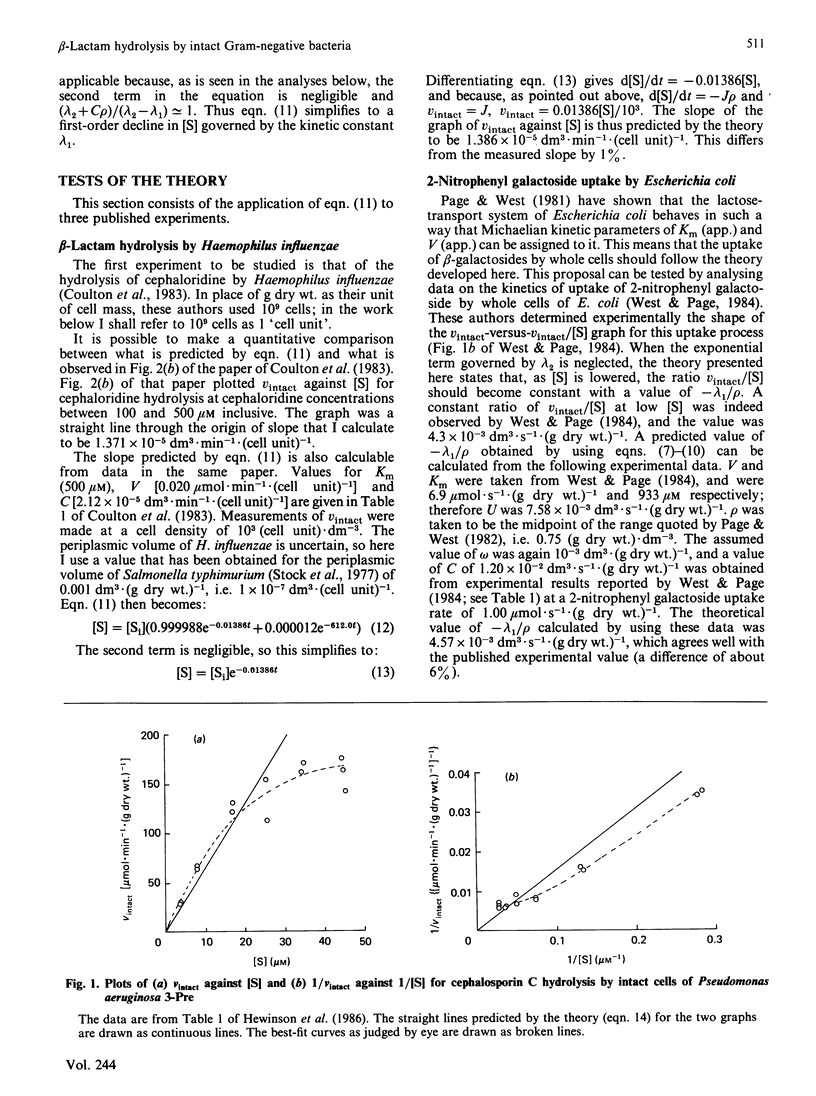
- Hewinson R. G., Lane D. C., Slack M. P., Nichols W. W. The permeability parameter of the outer membrane of Pseudomonas aeruginosa varies with the concentration of a test substrate, cephalosporin C. J Gen Microbiol. 1986 Jan;132(1):27–33. doi: 10.1099/00221287-132-1-27. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Normark S. Contribution of chromosomal beta-lactamases to beta-lactam resistance in enterobacteria. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S292–S304. doi: 10.1093/clinids/8.supplement_3.s292. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. G., Jou Y. H. Alternative-substrate inhibition and the kinetic mechanism of the beta-galactoside/proton symport of Escherichia coli. Biochem J. 1982 Jun 15;204(3):681–688. doi: 10.1042/bj2040681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. G., West I. C. The kinetics of the beta-galactoside-proton symport of Escherichia coli. Biochem J. 1981 Jun 15;196(3):721–731. doi: 10.1042/bj1960721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. An explicit model for bacterial resistance: application to beta-lactam antibiotics. Microbiol Sci. 1987 May;4(5):143–146. [PubMed] [Google Scholar]
- West I. C., Page M. G. When is the outer membrane of Escherichia coli rate-limiting for uptake of galactosides? J Theor Biol. 1984 Sep 7;110(1):11–19. doi: 10.1016/s0022-5193(84)80011-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


