Abstract
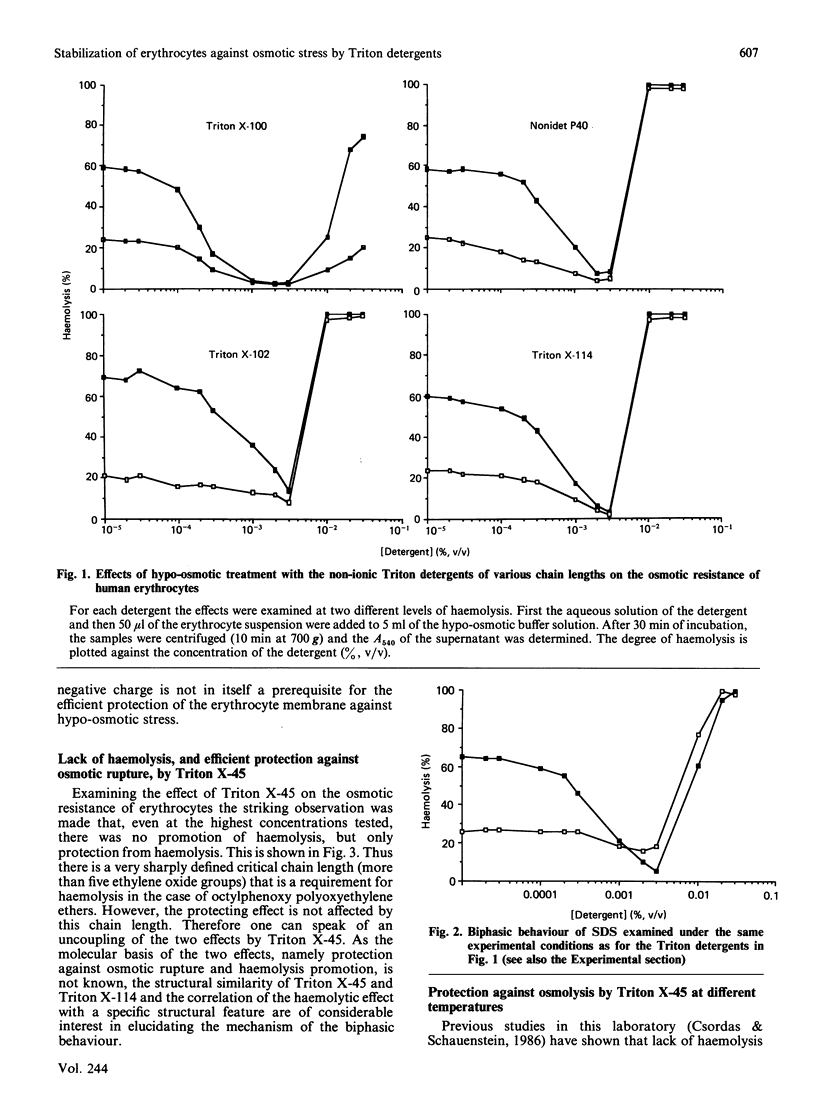
Octylphenoxy polyoxyethylene ethers (Triton detergents) interact with the erythrocyte membrane in a biphasic manner, i.e. they stabilize erythrocytes against hypo-osmotic haemolysis at low concentrations (0.0001-0.01%, v/v), but become haemolytic at higher concentrations. This biphasic behaviour was demonstrated with Triton X-114, Triton X-100 and Triton X-102. However, a critical chain length is a prerequisite for the haemolytic effect, because Triton X-45, which differs from the other Tritons only by the shorter chain of the polyoxyethylene residue, does not exhibit this biphasic behaviour, but goes on protecting against osmotic rupture up to saturating concentrations. Even a 1% solution of Triton X-45 does not cause haemolysis. This structural specificity of Triton X-45, namely the lack of haemolysis and efficient stabilization against osmolysis even at higher concentrations of the detergent, is exhibited at 0 degree and 37 degrees C as well as at room temperature. Three conclusions are reached: (i) a critical chain length of the octylphenoxy polyoxyethylene ethers is required for the haemolytic effect; (ii) the different structural requirements would suggest that different mechanisms are responsible for the haemolytic and the stabilizing effect of amphiphilic substances; (iii) the results suggest that haemolysis is not caused simply by dissolution of the membrane by the detergent but is a rather more specific process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969 Mar;21(1):73–103. [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Csordas A., Schauenstein K. Structure- and configuration-dependent effects of C18 unsaturated fatty acids on the chicken and sheep erythrocyte membrane. Biochim Biophys Acta. 1984 Feb 15;769(3):571–577. doi: 10.1016/0005-2736(84)90055-5. [DOI] [PubMed] [Google Scholar]
- Csordas A., Schauenstein K. Temperature-dependent specificity of cis-trans isomeric fatty acid interaction with the erythrocyte membrane. Biochim Biophys Acta. 1986 Apr 14;856(2):212–218. doi: 10.1016/0005-2736(86)90030-1. [DOI] [PubMed] [Google Scholar]
- Ehrly A. M., Gramlich F., Müller H. E. Die Bedeutung der unveresterten Fettsäuren für die Resistenz von Erythrozyten. Acta Haematol. 1964 Dec;32(6):348–354. doi: 10.1159/000209580. [DOI] [PubMed] [Google Scholar]
- Forte T., Leto T. L., Minetti M., Marchesi V. T. Protein 4.1 is involved in a structural thermotropic transition of the red blood cell membrane detected by a spin-labeled stearic acid. Biochemistry. 1985 Dec 31;24(27):7876–7880. doi: 10.1021/bi00348a005. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The membrane concentration of a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;183(3):530–543. doi: 10.1016/0005-2736(69)90167-9. [DOI] [PubMed] [Google Scholar]
- Kwant W. O., van Steveninck J. The influence of chlorpromazine on human erythrocytes. Biochem Pharmacol. 1968 Oct;17(10):2215–2223. doi: 10.1016/0006-2952(68)90196-2. [DOI] [PubMed] [Google Scholar]
- Le Maire M., Kwee S., Andersen J. P., Møller J. V. Mode of interaction of polyoxyethyleneglycol detergents with membrane proteins. Eur J Biochem. 1983 Jan 1;129(3):525–532. doi: 10.1111/j.1432-1033.1983.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Morariu V. V., Pop V. I., Popescu O., Benga G. Effects of temperature and pH on the water exchange through erythrocyte membranes: nuclear magnetic resonance studies. J Membr Biol. 1981;62(1-2):1–5. doi: 10.1007/BF01870194. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Reynolds J. A., Tanford C. The interaction of a cationic detergent with bovine serum albumin and other proteins. J Biol Chem. 1974 Jul 25;249(14):4452–4459. [PubMed] [Google Scholar]
- Podgórski A., Elbaum D. Properties of red cell membrane proteins: mechanism of spectrin and band 4.1 interaction. Biochemistry. 1985 Dec 31;24(27):7871–7876. doi: 10.1021/bi00348a004. [DOI] [PubMed] [Google Scholar]
- Ray A., Némethy G. Effects of ionic protein denaturants on micelle formation by nonionic detergents. J Am Chem Soc. 1971 Dec 15;93(25):6787–6793. doi: 10.1021/ja00754a014. [DOI] [PubMed] [Google Scholar]
- Raz A., Livne A. Differential effects of lipids on the osmotic fragility of erythrocytes. Biochim Biophys Acta. 1973 Jun 22;311(2):222–229. doi: 10.1016/0005-2736(73)90269-1. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., Seeman P. All lipid-soluble anaesthetics protect red cells. Nat New Biol. 1971 Jun 30;231(26):284–285. doi: 10.1038/newbio231284a0. [DOI] [PubMed] [Google Scholar]
- Rubin M. S., Tzagoloff A. Assembly of the mitochondrial membrane system. IX. Purification, characterization, and subunit structure of yeast and beef cytochrome oxidase. J Biol Chem. 1973 Jun 25;248(12):4269–4274. [PubMed] [Google Scholar]
- Seeman P. M. Membrane stabilization by drugs: tranquilizers, steroids, and anesthetics. Int Rev Neurobiol. 1966;9:145–221. doi: 10.1016/s0074-7742(08)60138-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]


