Abstract
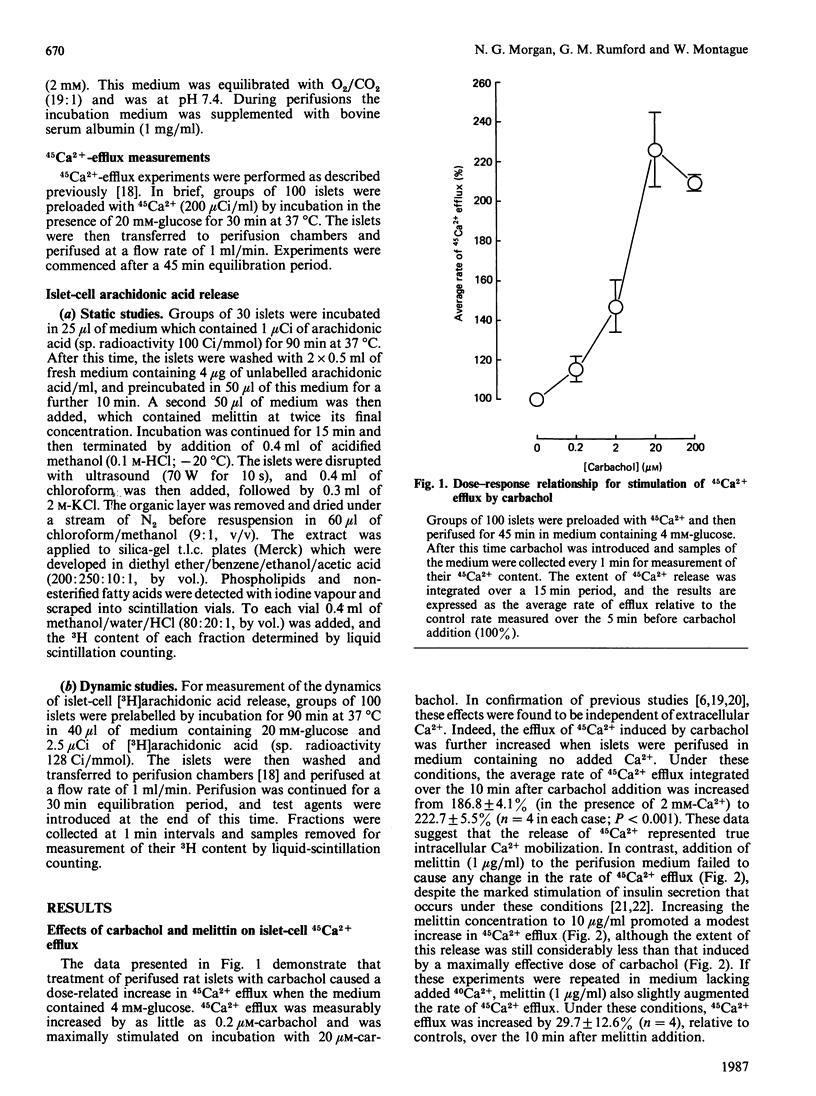
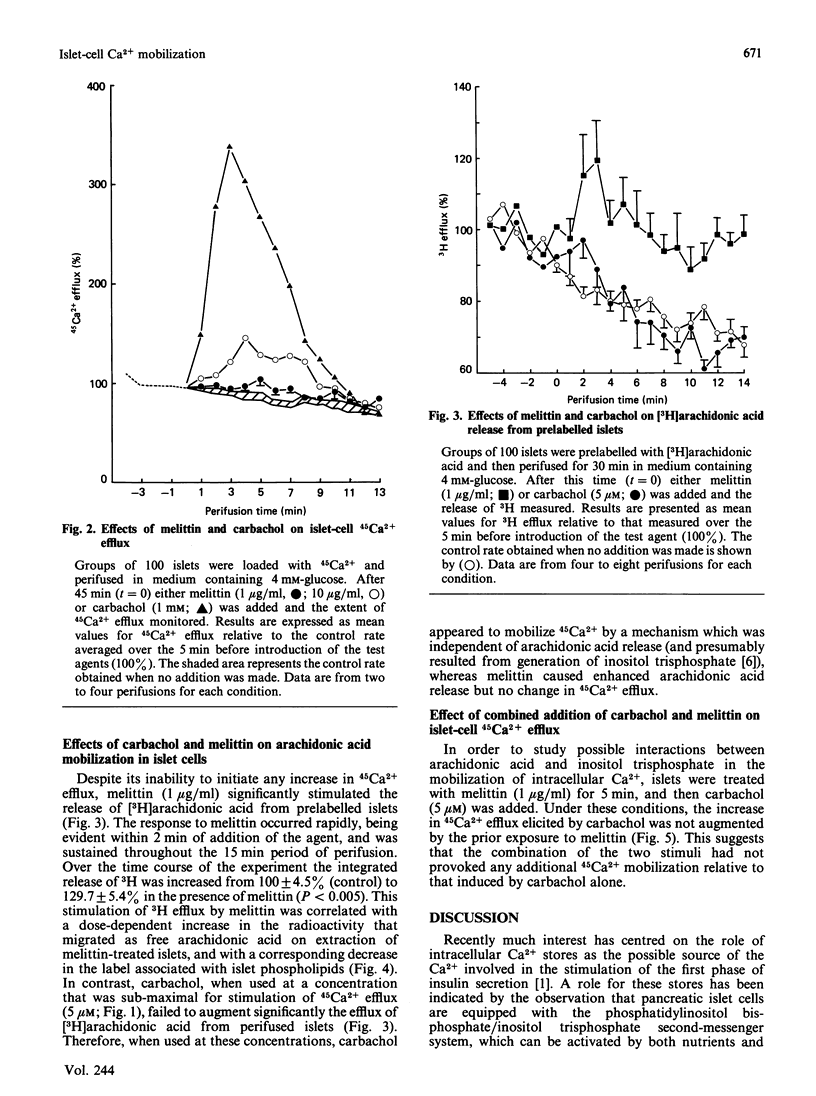
1. The rate of 45Ca2+ efflux from prelabelled rat islets of Langerhans was stimulated by carbachol in a dose-dependent manner. 2. Significant stimulation occurred in the presence of 0.2 microM-carbachol; the response was half-maximal at 3-5 microM and was maximal at 20 microM. 3. Stimulation of 45Ca2+ efflux by carbachol was not dependent on the presence of extracellular Ca2+ and was enhanced in Ca2+-depleted medium. 4. Stimulation of 45Ca2+ efflux by 5 microM-carbachol occurred independently of any change in [3H]arachidonic acid release in prelabelled islets, and probably reflected generation of inositol trisphosphate in the cells. 5. The amphipathic peptide melittin failed to increase islet-cell 45Ca2+ efflux at a concentration of 1 microgram/ml, and caused only a modest increase at 10 micrograms/ml. 6. Despite its failure to increase 45Ca2+ efflux, melittin at 1 microgram/ml caused a marked enhancement of 3H release from islets that had been prelabelled with [3H]arachidonic acid. 7. The stimulation of 3H efflux caused by melittin correlated with a dose-dependent increase in the unesterified [3H]arachidonic acid content of prelabelled islets and with a corresponding decrease in the extent of labelling of islet phospholipids. 8. Combined addition of melittin (1 microgram/ml) and 5 microM-carbachol to perifused islets failed to augment 45Ca2+ efflux relative to that elicited by carbachol alone. 9. The data indicate that melittin promotes an increase in arachidonic acid availability in intact rat islets. They do not, however, support the proposal that this can either directly reproduce or subsequently modify the extent of intracellular Ca2+ mobilization induced by agents that cause an increase in inositol trisphosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
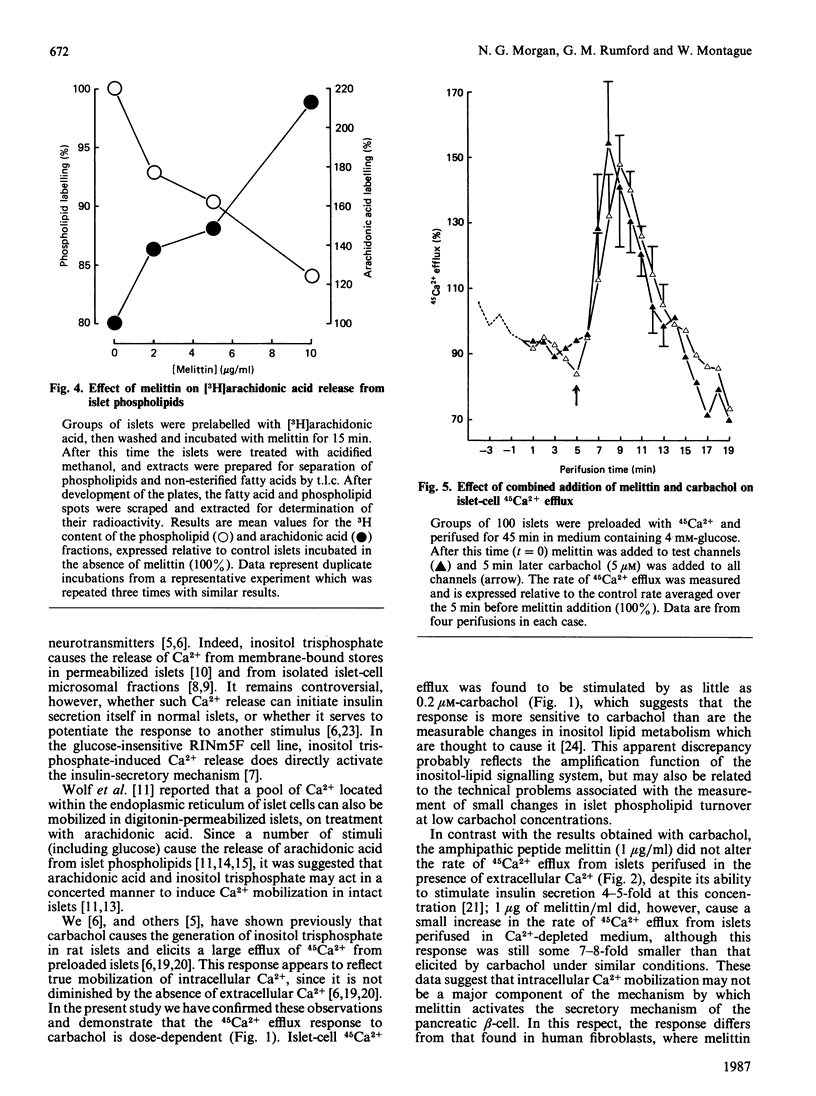
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984 Nov;115(5):1814–1820. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Phosphatidylinositol and phosphatidic acid metabolism in rat pancreatic islets in response to neurotransmitter and hormonal stimuli. Biochim Biophys Acta. 1983 Jan 7;750(1):157–163. doi: 10.1016/0005-2760(83)90215-1. [DOI] [PubMed] [Google Scholar]
- Hellman B., Gylfe E. Mobilization of different intracellular calcium pools after activation of muscarinic receptors in pancreatic beta-cells. Pharmacology. 1986;32(5):257–267. doi: 10.1159/000138178. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Kolesnick R. N., Gershengorn M. C. Arachidonic acid inhibits thyrotropin-releasing hormone-induced elevation of cytoplasmic free calcium in GH3 pituitary cells. J Biol Chem. 1985 Jan 25;260(2):707–713. [PubMed] [Google Scholar]
- Kolesnick R. N., Musacchio I., Thaw C., Gershengorn M. C. Arachidonic acid mobilizes calcium and stimulates prolactin secretion from GH3 cells. Am J Physiol. 1984 May;246(5 Pt 1):E458–E462. doi: 10.1152/ajpendo.1984.246.5.E458. [DOI] [PubMed] [Google Scholar]
- Laychock S. G. Fatty acid incorporation into phospholipids of isolated pancreatic islets of the rat. Relationship to insulin release. Diabetes. 1983 Jan;32(1):6–13. doi: 10.2337/diab.32.1.6. [DOI] [PubMed] [Google Scholar]
- Mathias P. C., Best L., Malaisse W. J. Stimulation by glucose and carbamylcholine of phospholipase A2 in pancreatic islets. Diabetes Res. 1985 Sep;2(5):267–270. [PubMed] [Google Scholar]
- Mathias P. C., Carpinelli A. R., Billaudel B., Garcia-Morales P., Valverde I., Malaisse W. J. Cholinergic stimulation of ion fluxes in pancreatic islets. Biochem Pharmacol. 1985 Oct 1;34(19):3451–3457. doi: 10.1016/0006-2952(85)90717-8. [DOI] [PubMed] [Google Scholar]
- Mix L. L., Dinerstein R. J., Villereal M. L. Mitogens and melittin stimulate an increase in intracellular free calcium concentration in human fibroblasts. Biochem Biophys Res Commun. 1984 Feb 29;119(1):69–75. doi: 10.1016/0006-291x(84)91619-x. [DOI] [PubMed] [Google Scholar]
- Montague W., Morgan N. G., Rumford G. M., Prince C. A. Effect of glucose on polyphosphoinositide metabolism in isolated rat islets of Langerhans. Biochem J. 1985 Apr 15;227(2):483–489. doi: 10.1042/bj2270483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Montague W. Stimulation of insulin secretion from isolated rat islets of Langerhans by melittin. Biosci Rep. 1984 Aug;4(8):665–671. doi: 10.1007/BF01121020. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Effects of noradrenaline on 45Ca2+ efflux from isolated rat islets of Langerhans. Biosci Rep. 1985 Dec;5(12):1053–1060. doi: 10.1007/BF01119626. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the mechanism by which melittin stimulates insulin secretion from isolated rat islets of Langerhans. Biochim Biophys Acta. 1985 Jun 30;845(3):526–532. doi: 10.1016/0167-4889(85)90221-6. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Short C. D., Rumford G. M., Montague W. Effects of the calcium-channel agonist CGP 28392 on insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Nov 1;231(3):629–634. doi: 10.1042/bj2310629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenquin M., Awouters P., Mathot F., Henquin J. C. Distinct effects of acetylcholine and glucose on 45calcium and 86rubidium efflux from mouse pancreatic islets. FEBS Lett. 1984 Oct 29;176(2):457–461. doi: 10.1016/0014-5793(84)81218-1. [DOI] [PubMed] [Google Scholar]
- Ostenson C. G., Grill V. Glucose exerts opposite effects on muscarinic receptor binding to A and B cells of the endocrine pancreas. Endocrinology. 1985 May;116(5):1741–1744. doi: 10.1210/endo-116-5-1741. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Goldsmith K. T. Phospholipase C and melittin enhancement of glucose-induced electrical activity. Endocrinology. 1986 Jan;118(1):102–107. doi: 10.1210/endo-118-1-102. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Mertz R. J., Kowluru A., Dixon J. F., Hokin L. E., MacDonald M. J. Evidence for glucose-responsive and -unresponsive pools of phospholipid in pancreatic islets. J Biol Chem. 1985 Jul 5;260(13):7861–7867. [PubMed] [Google Scholar]
- Renooij W., Van Golde L. M., Zwaal R. F., Van Deenen L. L. Topological asymmetry of phospholipid metabolism in rat erythrocyte membranes. Evidence for flip-flop of lecithin. Eur J Biochem. 1976 Jan 2;61(1):53–58. doi: 10.1111/j.1432-1033.1976.tb09996.x. [DOI] [PubMed] [Google Scholar]
- Turk J., Wolf B. A., McDaniel M. L. Glucose-induced accumulation of inositol trisphosphates in isolated pancreatic islets. Predominance of the 1,3,4-isomer. Biochem J. 1986 Jul 1;237(1):259–263. doi: 10.1042/bj2370259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. A., Comens P. G., Ackermann K. E., Sherman W. R., McDaniel M. L. The digitonin-permeabilized pancreatic islet model. Effect of myo-inositol 1,4,5-trisphosphate on Ca2+ mobilization. Biochem J. 1985 May 1;227(3):965–969. doi: 10.1042/bj2270965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]


