Abstract
Background
Iatrogenic bile duct injuries (BDIs) prevention during laparoscopic cholecystectomy (LC) relies on meticulous anatomical dissections through direct visualization. Near-infrared fluorescence (NIRF) with indocyanine green (ICG) improves the visualization of extrahepatic biliary structures. Although ICG can be administered either intravenously or intragallbladder, there remains uncertainty regarding the optimal method for different patient populations. This study sought to assess the suitability of each method for specific patient groups.
Methods
Between October 2021 and May 2022, 59 consecutive patients underwent fluorescence-guided LC at West China Hospital of Sichuan University. Among them, 32 patients received an intravenous injection of ICG (10 mg) 10 to 12 hours prior to surgery (Group A: the intravenous group), while 27 patients received an intragallbladder injection of ICG (10 mg) (Group B: the intragallbladder group). Baseline clinical factors, inclusion criteria, and measurements of parameters and complications were assessed. Data were retrospectively collected and analyzed to evaluate the comparability of the two groups and the clinical outcomes.
Results
Groups A and B included 32 patients (18 males, 14 females), and 27 patients (13 men, 14 women), respectively. In our statistical analysis, significant differences were observed in preoperative diagnoses between the two groups (P=0.041), but the majority of other baseline clinical factors were comparable. Notably, no statistically significant differences were found in complication rates. However, Group A had a shorter operative time (60.38±9.35 vs. 66.78±9.88 min, P=0.01) and superior bile duct fluorescence (P=0.04) than Group B. Interestingly, fluorescence was not observed in impacted gallbladder stones in Group B. Additionally, patients with cirrhosis (P=0.008) and fatty liver (P=0.005) in Group B had higher common bile duct-to-liver ratios (BLRs) than those in Group A.
Conclusions
ICG fluorescence cholangiography allows to visualize extrahepatic biliary anatomical structures with both administration methods. However, the efficacy of bile duct fluorescence varies with different administration routes in diverse patient populations. Hence, appropriate administration route selection for ICG should be tailored to individual patients.
Keywords: Fluorescence cholangiography, indocyanine green (ICG), near-infrared, laparoscopic cholecystectomy (LC)
Highlight box.
Key findings
• Near-infrared fluorescence (NIRF) with indocyanine green (ICG) effectively visualizes extrahepatic biliary structures when administered both intravenously and directly in the gallbladder. However, the fluorescence efficacy for bile ducts varies across patient populations and administration methods.
What is known, and what is new?
• NIRF with ICG cholangiography (NIFC) was significantly superior to white light alone in enhancing the visualization of extrahepatic biliary structures during laparoscopic cholecystectomy.
• There is a lack of comparative studies concerning the two administration routes (intravenous and intragallbladder) of ICG. This retrospective study investigated the effects of different ICG administration routes for common bile duct fluorescence imaging.
What is the implication, and what should change now?
• Preoperative intravenous ICG injection is safer than intragallbladder injection. However, in selected patients with concomitant cirrhosis or fatty liver, given the potential interference of liver background fluorescence, we recommend, when possible, intragallbladder injection.
Introduction
Laparoscopic cholecystectomy (LC) is the minimally invasive surgical procedure for the gallbladder removal. It is estimated that approximately 3,000,000 LCs are performed in the United States annually (1). Notably, iatrogenic bile duct injury (BDI) is a significant complication of LC, with an incidence rate ranging from 0.3% to 0.7% (2-4). The most common reason for BDI is the misidentification of the biliary anatomy (2). To address the risk of iatrogenic BDI during LC, Strasberg introduced the “Critical View of Safety” concept in 1995 (5). This concept has been increasingly recognized as the standard method for the cystic duct and artery identification to mitigate BDI risk and prevent errors stemming from anatomical deviations (6). In subsequent years, the use of techniques such as intraoperative ultrasound and intraoperative cholangiography have been suggested during LC (7). Nevertheless, the adoption of these methods in the clinical practice has been limited due to constraints such as time consumption, surgeons’ experience, increased costs and radiation exposure (8).
Recent research has shown the efficacy of the near-infrared fluorescence (NIRF) with indocyanine green (ICG) in visualizing the extrahepatic biliary structures (9-11). Dip et al. (12) showed that NIRF with ICG cholangiography (NIFC) was significantly superior to white light in enhancing the visualization of extrahepatic biliary structures during LC. Our preliminary study also confirmed that the preoperative intravenous injection of 10 mg ICG, 10 to 12 hours before surgery, yielded satisfactory imaging results (13). However, this approach requires nighttime administration, with inconveniences both for patients and healthcare providers. Additionally, if patients have factors impeding ICG metabolism or excretion, the peripheral intravenous injection of ICG may not yield optimal biliary fluorescence visualization (14,15). Conversely, the intragallbladder ICG injection effectively circumvents interference from hepatic background fluorescence on biliary fluorescence visualization (16). Moreover, the timing of this ICG administration approach is more convenient than that of a preoperative peripheral intravenous injection (16). Liu et al. (17) explored the technique of intragallbladder ICG injection and found that NIFC can achieve noise-free visualization of the biliary anatomy.
Although existing literature has documented favorable results in visualizing the extrahepatic bile duct for each method, there remains uncertainty regarding the optimal method for different patient populations. Furthermore, there is a lack of comparative studies on the two ICG administration routes. The aim of this retrospective study is to explore the optimal ICG administration route for patients who underwent LC using NIRF. We present this article in accordance with the STROBE reporting checklist (18) (available at https://gs.amegroups.com/article/view/10.21037/gs-24-198/rc).
Methods
Study design and participants
Patient data were collected from individuals who underwent LC at the Department of Pancreatic Surgery, West China Hospital of Sichuan University, between October 2021 and May 2022. The study was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University [No. 2023 Review (1593)]. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for written informed consent was waived by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University due to the retrospective nature of the study. The enrolled patients received an ICG injection and underwent intraoperative NIRF imaging. Patients without surgical videos were excluded. Based on the method of ICG administration, patients were divided into the following two groups: the peripheral intravenous injection group (Group A), and the intragallbladder injection group (Group B). In Group A, 10 mg of ICG were administered via peripheral intravenous injection 10 to 12 hours before surgery. In Group B, 3–5 mL of normal saline containing 10 mg of ICG were injected into the gallbladder lumen during surgery.
Data on patient characteristics were collected, including baseline demographics, intraoperative outcomes, and postoperative complications. Baseline characteristics included age, gender, indication to surgery, total serum bilirubin, direct serum bilirubin, comorbidities, and impacted gallbladder stones (yes/no). The intraoperative outcomes and postoperative complications, including operative time (min), blood loss (mL), and gallbladder difficulty scores [assessed according to Tokyo Guidelines (19)]. The BDI occurrence, bile leakage, and postoperative pancreatitis were also assessed. Data on common bile duct fluorescence visualization and the common bile duct-to-liver ratio (BLR) were collected. The Image-Pro Plus software (developed by Media Cybernetics Corporation, Rockville, USA) was used to measure the fluorescence intensity of the common bile duct and liver and to subsequently calculate the BLR. To address potential sources of bias, our study implemented the following measures: first, we minimized selection bias by employing stringent inclusion and exclusion criteria; second, data collection was conducted by trained researchers, ensuring a standardized process to reduce information bias.
Statistical analysis
Statistical analysis was performed using IBM SPSS 19.0 (SPSS Inc., Chicago, IL, USA). If there is minimal missing data, we opt to delete the observations containing missing values. The normally distributed continuous variables were expressed as mean ± standard deviation (SD) and were analyzed using the independent sample t-test. The non-normally distributed continuous variables were expressed as median (interquartile range), and group differences were compared using the Mann-Whitney U test. The categorical variables are expressed as frequency (n, %), and were analyzed using the Chi-squared or Fisher’s exact test. All the tests were two-sided, with a significance level set at P<0.05.
Results
Patients clinical characteristics
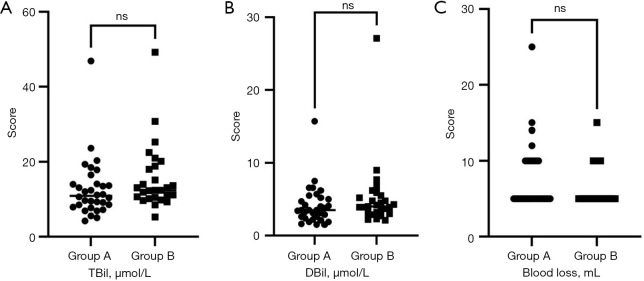
The clinical characteristics of the two groups are reported in Table 1. Group A included 32 patients, of whom 18 men and 14 women. Group B included 27 patients, of whom 13 men and 14 women (P=0.54). Mean age of patients in Group A was 51.16 years, while in Group B was 47.04 years (P=0.17). There were no statistically significant differences between the two groups in terms of number of patients with cirrhosis (P=0.72), fatty liver (P=0.74), preoperative total serum bilirubin (Figure 1A, P=0.07), direct serum bilirubin (Figure 1B, P=0.14), and impacted gallbladder stones (P=0.65). However, there was a statistically significant difference between the two groups in terms of preoperative diagnosis (P=0.04).
Table 1. Patients’ clinical characteristics at the baseline.
| Demographics | Group A (n=32) | Group B (n=27) | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 51.16±12.52 | 47.04±9.93 | 0.17 |
| Sex, n (%) | 0.54 | ||
| Male | 18 (56.25) | 13 (48.15) | |
| Female | 14 (43.75) | 14 (51.85) | |
| Preoperative diagnosis, n (%) | 0.04 | ||
| Gallbladder polyps | 7 (21.88) | 12 (44.44) | |
| Single stone of the gallbladder | 9 (28.12) | 9 (33.33) | |
| Multiple stones in the gallbladder | 15 (46.88) | 4 (14.82) | |
| Gallbladder adenomyosis | 1 (3.12) | 2 (7.41) | |
| Comorbidities, n (%) | |||
| Cirrhosis | 4 (12.50) | 5 (18.52) | 0.72 |
| Fatty liver | 6 (18.75) | 4 (14.82) | 0.74 |
| Biochemical indexes, median [range] | |||
| TBil (μmol/L) | 10.95 [4, 47] | 12.40 [5, 49] | 0.07 |
| DBil (μmol/L) | 3.5 [2, 16] | 4 [2, 27] | 0.14 |
| Gallbladder duct stone impaction, n (%) | 2 (6.25) | 3 (11.11) | 0.65 |
Group A, the patients received ICG via intravenous injection; Group B, the patients received ICG via intragallbladder injection. SD, standard deviation; TBil, total bilirubin; DBil, direct bilirubin; ICG, indocyanine green.
Figure 1.
No statistically significant differences were observed between the two groups regarding preoperative levels of total bilirubin (A, P=0.07, Mann-Whitney U test), direct bilirubin (B, P=0.14, Mann-Whitney U test), or blood loss (C, P=0.18, Mann-Whitney U test). Group A, patients received ICG via intravenous injection; Group B patients received ICG via intragallbladder injection. ns, non-significant; TBil, total bilirubin; DBil, direct bilirubin; ICG, indocyanine green.
Surgical outcomes
The surgical outcomes are reported in Table 2. The mean operative time of Group B was 6.4 min longer than that of Group A (66.78 vs. 60.38 min; P=0.01). There were no statistically significant differences between the two groups in terms of blood loss (Figure 1C, P=0.18), gallbladder difficulty scores (P>0.99), bile leakage (P>0.99), BDI rate (P>0.99), and postoperative pancreatitis (P=0.46). One patient in Group B developed postoperative pancreatitis. This patient had multiple gallstones before surgery. The patient showed improvement following symptomatic supportive treatments, including antibiotic therapy, suppression of pancreatic secretion, and fasting. On the fifth postoperative day, amylase levels returned into normal range.
Table 2. Surgical outcomes.
| Surgical outcomes | Group A (n=32) | Group B (n=27) | P value |
|---|---|---|---|
| Gallbladder difficulty scores, n (%) | >0.99 | ||
| 2 | 24 (75.00) | 20 (74.07) | |
| 3 | 7 (21.88) | 7 (25.93) | |
| 4 | 1 (3.12) | 0 | |
| Operative time (min) (mean ± SD) | 60.38±9.35 | 66.78±9.88 | 0.01 |
| Bile leak | 0 | 0 | >0.99 |
| BDI | 0 | 0 | >0.99 |
| Pancreatitis, n (%) | 0 | 1 (3.70) | 0.46 |
| Blood loss (mL), median [range] | 5 [5, 25] | 5 [5, 15] | 0.18 |
Group A, the patients received ICG via intravenous injection; Group B, the patients received ICG via intragallbladder injection. SD, standard deviation; BDI, bile duct injury; ICG, indocyanine green.
Comparison of common bile duct fluorescence visualization between the two patient groups
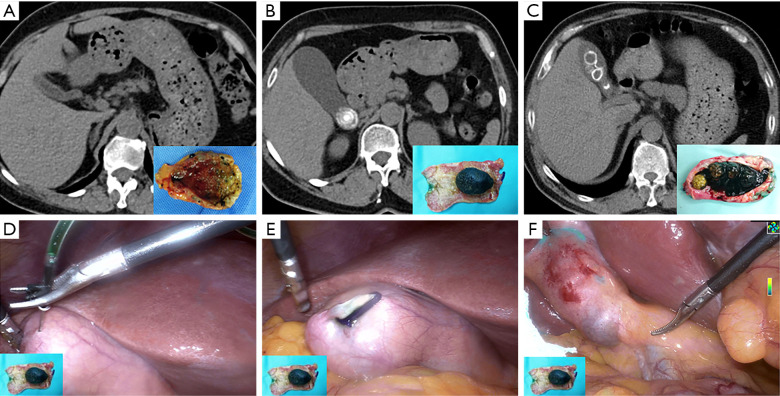
The results of common bile duct fluorescence visualization in the two groups are reported in Table 3. All the patients in Group A exhibited common bile duct fluorescence visualization, while in Group B, common bile duct fluorescence was not observed in four patients. The Fisher’s exact test results revealed a statistically significant difference between the two groups in terms of common bile duct fluorescence visualization (P=0.04). Among these four patients, one had gallbladder stones combined with gallbladder atrophy (Figure 2A), while the other three patients had impacted gallbladder stones (Figure 2B-2F). There was no statistically significant difference in common bile duct fluorescence visualization among patients with impacted gallbladder stones between the two groups (P=0.10); all three patients in Group B failed to show fluorescence visualization, while both patients in Group A exhibited fluorescence visualization. Examples of extrahepatic biliary fluorescence visualization in the two patient groups are reported in Videos S1,S2.
Table 3. Intraoperative fluorescence cholangiography.
| Common bile duct fluorescence imaging | Group A (n=32) | Group B (n=27) | P value | |||
|---|---|---|---|---|---|---|
| Visible | Not visible | Visible | Not visible | |||
| Diagnosis, n (%) | 32 (100.00) | 0 | 23 (85.19) | 4 (14.81) | 0.04 | |
| Single stone of the gallbladder | 9 | 0 | 7 | 2 | ||
| Multiple stones in the gallbladder | 15 | 0 | 2 | 2 | ||
| Gallbladder polyps | 7 | 0 | 12 | 0 | ||
| Gallbladder adenomyosis | 1 | 0 | 2 | 0 | ||
| Gallbladder duct stone impaction, n (%) | 2 (100.00) | 0 | 0 | 3 (100.00) | 0.10 | |
Group A, the patients received ICG via intravenous injection; Group B, the patients received ICG via intragallbladder injection. ICG, indocyanine green.
Figure 2.
Preoperative computed tomography images and intraoperative common bile duct fluorescence imaging. (A) Gallbladder stone combined with gallbladder atrophy; (B) single impacted gallbladder stone impaction; (C) multiple gallbladder stones and gallbladder stone impaction; (D-F) common bile duct fluorescence imaging results after gallbladder injection of ICG in patients with single gallbladder stone impaction. ICG, indocyanine green.
Video S1.
Fluorescent cholangiography of extrahepatic biliary system following intravenous ICG injection, 90 s, 73 MB. The patient was a 55-year-old man with a gallbladder polyp. He was injected with 10 mg of ICG via the peripheral vein 10 h prior to surgery. Before LC, his total serum bilirubin was 11.3 µmol/L. The operative time was 61 min, and the blood loss was 15 mL. His postoperative course was uneventful.
Video S2.
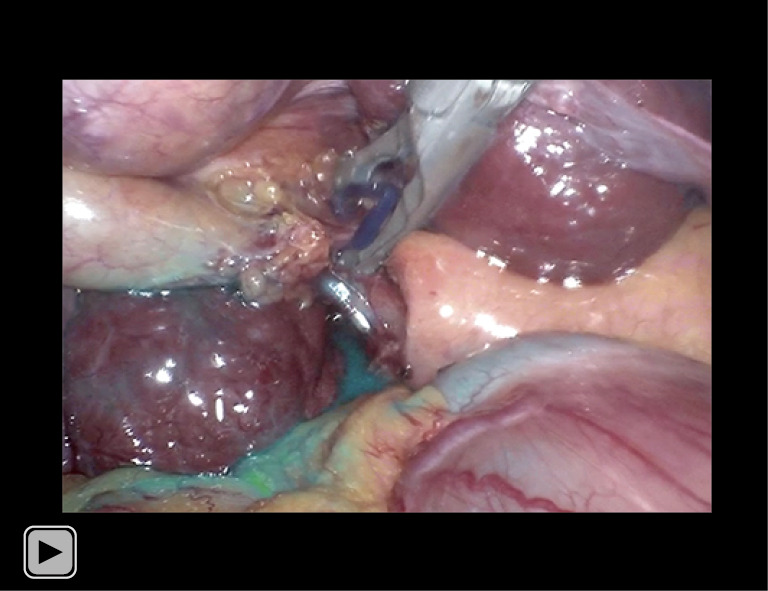
Fluorescent cholangiography of extrahepatic biliary system following intragallbladder ICG injection, 51 s, 40.6 MB. The patient was a 62-year-old male with a solitary gallbladder stone combined with liver cirrhosis. Intraoperatively, 4 mL of saline containing 10 mg of ICG was injected through the gallbladder. Before cholecystectomy, his serum total bilirubin was 21.0 µmol/L. The operative time was 72 min, and the blood loss was 20 mL. Postoperative recovery was uneventful.
Comparison of the BLRs between the two patient groups
The fluorescence intensity ratio between the common bile duct and the liver (i.e., the BLR) is a crucial parameter for assessing the efficacy of common bile duct fluorescence visualization (20-22). We also employed this ratio in our previous research to evaluate the effect of common bile duct fluorescence imaging (13). In this study, we calculated the BLRs of the two groups of patients with different diagnosis. Interestingly, we did not find statistically significant differences in the BLRs among patients with solitary gallbladder stones (P>0.99), multiple gallbladder stones (P=0.60), or gallbladder polyps (P=0.37). The BLR values of patients with gallbladder adenomyomatosis in both groups were consistently above 1 (Table 4).
Table 4. The fluorescence intensity of the common BLR.
| Patients’ clinical characteristics | Group A | Group B | P value | |||
|---|---|---|---|---|---|---|
| BLR >1 | BLR ≤1 | BLR >1 | BLR ≤1 | |||
| Preoperative diagnosis, n (%) | 23 (71.87) | 9 (28.13) | 23 (85.19) | 4 (14.81) | 0.35 | |
| Single stone of the gallbladder | 6 (66.67) | 3 (33.33) | 7 (77.78) | 2 (22.22) | >0.99 | |
| Multiple stones in the gallbladder | 10 (66.67) | 5 (33.33) | 2 (50.00) | 2 (50.00) | 0.60 | |
| Gallbladder polyps | 6 (85.71) | 1 (14.29) | 12 (100.00) | 0 | 0.37 | |
| Gallbladder adenomyosis | 1 (100.00) | 0 | 2 (100.00) | 0 | ||
| Comorbidities, n (%) | ||||||
| Cirrhosis | 0 | 4 (100.00) | 5 (100.00) | 0 | 0.008 | |
| Fatty liver | 0 | 6 (100.00) | 4 (100.00) | 0 | 0.005 | |
| Gallbladder duct stone impaction, n (%) | 1 (50.00) | 1 (50.00) | 0 | 3 (100.00) | 0.40 | |
Group A, the patients received ICG via intravenous injection; Group B, the patients received ICG via intragallbladder injection. BLR, bile duct-to-liver ratio; ICG, indocyanine green.
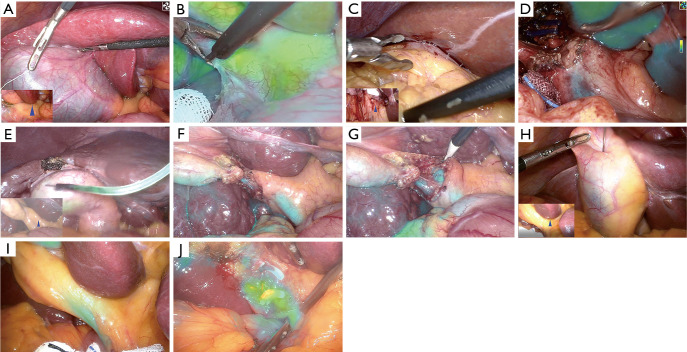
Compared to patients receiving intravenous ICG injection (Figure 3A-3D), patients with concurrent liver cirrhosis or fatty liver who received a ICG gallbladder injection (Figure 3E-3J) achieved superior discriminatory common bile duct fluorescence imaging results. A further statistical analysis was conducted of the BLRs of both groups of patients with concurrent liver cirrhosis or fatty liver, and statistically significant differences were observed in the BLRs of the two groups among patients with liver cirrhosis (P=0.008) and fatty liver (P=0.005) (Table 4). Despite statistically significant differences were not observed in the BLRs of patients with impacted gallbladder stones between the two groups, it should be noted that among patients in Group B with impacted gallbladder stones, all the BLR values were consistently below 1.
Figure 3.
Fluorescence imaging results in patients with either cirrhosis or fatty liver in Group A and Group B. (A) Patients with combined cirrhosis in Group A; (B) fluorescence imaging results of patients with combined cirrhosis in Group A; (C) patients with combined fatty liver in Group A; (D) fluorescence imaging results of patients with combined fatty liver in Group A; (E) patients with combined cirrhosis in Group B; (F,G) fluorescence imaging results of patients with combined cirrhosis in Group B; (H) patients with combined fatty liver in Group B; (I,J) fluorescence imaging results in patients with combined fatty liver in Group B. The blue arrowheads represent hepatoduodenal ligament.
Discussion
This study investigated the effects of different ICG administration routes on common bile duct fluorescent imaging. Through a retrospective analysis of 59 patients, we observed that the common bile duct fluorescence visualization rates were 100% in the peripheral venous ICG injection group and 85.19% in the gallbladder ICG injection group (P=0.04). A further analysis of the reasons behind this discrepancy revealed that in all patients with impacted gallbladder stones, common bile duct fluorescence imaging could not be achieved through gallbladder ICG injection. Additionally, the proportions of patients in both groups with a BLR greater than 1 were 71.87% and 85.19%, respectively (P=0.35). A further analysis of patients with combined liver cirrhosis or fatty liver revealed that the proportions of patients with a BLR greater than 1 in these groups were 0% and 100%, respectively. Furthermore, we observed that a patient developed postoperative pancreatitis after intragallbladder ICG injection. This patient presented with multiple gallstones and sediment-like calculi in the gallbladder. Our analysis suggests that the intraoperative displacement of sediment-like calculi into the common bile duct might have contributed to this incidence of pancreatitis. Consequently, for patients with impacted gallbladder stones or sediment-like gallbladder stones, preoperative ICG injection is recommended. Conversely, for patients with cirrhosis or fatty liver, an intraoperative gallbladder ICG injection could enhance discernibility for better visualization.
Recently, the application of ICG fluorescence cholangiography in liver extrahepatic biliary surgery has expanded. The refinement of this emerging technique could significantly influence the pivotal progress of future research in this domain (23). A recent multicenter prospective randomized controlled trial shed light on the early identification of pertinent extrahepatic biliary anatomical structures during LC through the application of NIRF imaging (24). This advanced imaging technique facilitates the prompt visualization of critical components, such as the cystic duct, common bile duct, and the transition from the cystic duct artery to the gallbladder. The quest to optimize the fluorescence imaging effect of extrahepatic bile duct has been a persistent focus of researchers (9,13,21,22,25). The administration timing (9,13,22,25), dosage (9,21,25), and delivery routes (16,26) of ICG have all been subjects of corresponding investigational reports. In this study, a particular emphasis is placed on exploring the delivery routes for ICG. The findings of this research underscore the importance of tailoring the choice of ICG administration route based on individual patient characteristics to achieve the desired optimal fluorescent cholangiography outcomes.
Previous studies have compared the two routes of ICG administration; however, research on the selection of the optimal ICG administration route is limited. Shibata et al. (26) elucidated the differences between intravenous ICG injection and intrabiliary ICG injection. However, three distinct methods were used in the intrabiliary administration group. Specifically, eight patients received ICG via hepaticocholecystic drainage, one through endoscopic nasobiliary drainage, and three through direct gallbladder injection. Due to the limited sample size and the multiple intrabiliary injection methods, a conclusive determination favoring one approach over another one has yet to be determined. A case-control study by Castagneto-Gissey et al. (16) explored the fluorescence cholangiography effects of the two ICG injection methods and found that both injection methods aided in the anatomical delineation of Calot triangle for extrahepatic biliary structures. In comparison to intravenous ICG injection, the intragallbladder ICG administration route mitigated liver fluorescence, resulting in a superior signal-to-noise ratio and heightened contrast between bile ducts and liver tissue. Additionally, they noted that peripheral venous administration was more effective in outlining the duodenum and common hepatic duct than gallbladder puncture administration. Both studies affirmed the efficacy of both injection methods for extrahepatic bile duct fluorescent imaging. However, a definitive answer as to which method to choose for ICG administration based on patient characteristics needs to further studies.
Based on our research findings, we recommend that an individualized approach is adopted to select the most suitable ICG administration route based on each patient’s unique characteristics (Table 5). Intraoperative fluorescence cholangiography using intragallbladder ICG injection should be avoided in case of patient with: (I) multiple gallstones or a gallbladder filled with stones; (II) sediment-like gallbladder stones; (III) impacted gallbladder stones; (IV) potentially malignant gallbladder polyps; (V) gallbladder atrophy; and/or (VI) liver duct anatomy variations. The preoperative intravenous ICG injection is a safer alternative than the intragallbladder ICG injection. However, in case of patients with concomitant cirrhosis or fatty liver, given the potential interference of liver background fluorescence, we recommend that, when the option is available, intragallbladder ICG injection is used.
Table 5. Comparison of the two methods of ICG injection.
| Intravenous injection via peripheral veins |
| Indications: |
| Gallbladder stone impaction |
| Gallbladder filled with stones |
| Gallbladder polyps |
| Gallbladder atrophy |
| Gallbladder polyps with potential malignancy |
| Gallbladder adenomyomatosis |
| Preoperative intravenous injection of ICG may not achieve optimal contrast enhancement in patients with: |
| Liver cirrhosis |
| Fatty liver |
| Percutaneous injection via gallbladder |
| Indications: |
| Patients with liver cirrhosis |
| Patients with fatty liver |
| Gallbladder polyps without potential malignancy |
| Single and freely movable gallbladder stone |
| Gallbladder adenomyomatosis |
| No risk of substances in the gallbladder dropping into the common bile duct after injection (e.g., gallbladder sandy stone) |
| This injection method is not suitable if the patient has: |
| Variant accessory hepatic ducts or aberrant hepatic ducts, as this method may not be suitable for fluorescence imaging |
| Gallbladder stone impaction |
| Gallbladder filled with stones |
| Gallbladder polyps with potential malignancy |
| Gallbladder atrophy |
ICG, indocyanine green.
The main limitations of the present study are its retrospective nature and the sample size.
Conclusions
ICG fluorescence cholangiography allows to visualize extrahepatic biliary anatomical structures under both administration methods. However, the efficacy of bile duct fluorescence varies with different administration routes in diverse patient populations. Our investigation revealed that patients with gallbladder atrophy, sediment-like gallstones, fully occupied gallstones, or impacted stones in the gallbladder duct or neck were not suitable candidates for intragallbladder ICG injection. In our opinion, for such patients, intravenous ICG injection is recommended. Patients with comorbidities such as cirrhosis or fatty liver tend to exhibit stronger hepatic background fluorescence with intravenous ICG injection. Hence, for this subgroup of patients, intraoperative gallbladder injection of ICG may be a more suitable option. Hence, appropriate administration route selection for ICG should be tailored to individual patients.
Further studies with large sample of patients are required to confirm our results.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by a grant from the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. 2018HXFH015).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University [No. 2023 Review (1593)]. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for written informed consent was waived by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University due to the retrospective nature of the study.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-198/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-198/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
Data Sharing Statement
Available at https://gs.amegroups.com/article/view/10.21037/gs-24-198/dss
References
- 1.Hassler KR, Collins JT, Philip K, et al. Laparoscopic Cholecystectomy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; January 23, 2023. [PubMed] [Google Scholar]
- 2.Pesce A, Palmucci S, La Greca G, et al. Iatrogenic bile duct injury: impact and management challenges. Clin Exp Gastroenterol 2019;12:121-8. 10.2147/CEG.S169492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez JV, Chen DG, Yheulon CG, et al. Acute cholecystitis, obesity, and steatohepatitis constitute the lethal triad for bile duct injury (BDI) during laparoscopic cholecystectomy. Surg Endosc 2024;38:2475-82. Erratum in: Surg Endosc 2024;38:2911. 10.1007/s00464-024-10727-9 [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Singh J, Mishra A, et al. Efficacy and outcome of indocyanine green-based intraoperative cholangiography using near-infrared fluorescence imaging: A prospective study. J Minim Access Surg 2024;20:89-95. 10.4103/jmas.jmas_228_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995;180:101-25. [PubMed] [Google Scholar]
- 6.Manatakis DK, Antonopoulou MI, Tasis N, et al. Critical View of Safety in Laparoscopic Cholecystectomy: A Systematic Review of Current Evidence and Future Perspectives. World J Surg 2023;47:640-8. 10.1007/s00268-022-06842-0 [DOI] [PubMed] [Google Scholar]
- 7.Glaysher MA, Beable R, Ball C, et al. Intra-operative ultrasound assessment of the biliary tree during robotic cholecystectomy. J Robot Surg 2023;17:2611-5. 10.1007/s11701-023-01701-z [DOI] [PubMed] [Google Scholar]
- 8.Quaresima S, Balla A, Palmieri L, et al. Routine near infra-red indocyanine green fluorescent cholangiography versus intraoperative cholangiography during laparoscopic cholecystectomy: a case-matched comparison. Surg Endosc 2020;34:1959-67. 10.1007/s00464-019-06970-0 [DOI] [PubMed] [Google Scholar]
- 9.Baldari L, Boni L, Kurihara H, et al. Identification of the ideal weight-based indocyanine green dose for fluorescent cholangiography. Surg Endosc 2023;37:7616-24. 10.1007/s00464-023-10280-x [DOI] [PubMed] [Google Scholar]
- 10.She WH, Cheung TT, Chan MY, et al. Routine use of ICG to enhance operative safety in emergency laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc 2022;36:4442-51. 10.1007/s00464-021-08795-2 [DOI] [PubMed] [Google Scholar]
- 11.Reeves JJ, Broderick RC, Lee AM, et al. The price is right: Routine fluorescent cholangiography during laparoscopic cholecystectomy. Surgery 2022;171:1168-76. 10.1016/j.surg.2021.09.027 [DOI] [PubMed] [Google Scholar]
- 12.Dip F, LoMenzo E, Sarotto L, et al. Randomized Trial of Near-infrared Incisionless Fluorescent Cholangiography. Ann Surg 2019;270:992-9. 10.1097/SLA.0000000000003178 [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Zhou R, Weng J, et al. Extrahepatic biliary tract visualization using near-infrared fluorescence imaging with indocyanine green: optimization of dose and dosing time. Surg Endosc 2021;35:5573-82. 10.1007/s00464-020-08058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CHERRICK GR , STEIN SW, LEEVY CM, et al. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest 1960;39:592-600. 10.1172/JCI104072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015;29:368-75. 10.1007/s00464-014-3677-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castagneto-Gissey L, Russo MF, Iodice A, et al. Intracholecystic versus Intravenous Indocyanine Green (ICG) Injection for Biliary Anatomy Evaluation by Fluorescent Cholangiography during Laparoscopic Cholecystectomy: A Case-Control Study. J Clin Med 2022;11:3508. 10.3390/jcm11123508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YY, Liao CH, Diana M, et al. Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: preliminary clinical results. Surg Endosc 2018;32:1506-14. 10.1007/s00464-017-5838-9 [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi G, Iwashita Y, Hibi T, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci 2018;25:73-86. 10.1002/jhbp.517 [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Chen Q, Kuang J, et al. Real-time fluorescent cholangiography with indocyanine green in laparoscopic cholecystectomy: a randomized controlled trial to establish the optimal indocyanine green dose within 30 min preoperatively. Surg Today 2023;53:223-31. 10.1007/s00595-022-02563-y [DOI] [PubMed] [Google Scholar]
- 21.Zarrinpar A, Dutson EP, Mobley C, et al. Intraoperative Laparoscopic Near-Infrared Fluorescence Cholangiography to Facilitate Anatomical Identification: When to Give Indocyanine Green and How Much. Surg Innov 2016;23:360-5. 10.1177/1553350616637671 [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui N, Yoshida M, Nakagawa H, et al. Optimal timing of preoperative indocyanine green administration for fluorescent cholangiography during laparoscopic cholecystectomy using the PINPOINT® Endoscopic Fluorescence Imaging System. Asian J Endosc Surg 2018;11:199-205. 10.1111/ases.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo D, Liang W, Ma B, et al. Global trends of indocyanine green fluorescence navigation in laparoscopic cholecystectomy: bibliometrics and knowledge atlas analysis. Surg Endosc 2022;36:6419-31. 10.1007/s00464-021-08988-9 [DOI] [PubMed] [Google Scholar]
- 24.van den Bos J, Schols RM, Boni L, et al. Near-infrared fluorescence cholangiography assisted laparoscopic cholecystectomy (FALCON): an international multicentre randomized controlled trial. Surg Endosc 2023;37:4574-84. 10.1007/s00464-023-09935-6 [DOI] [PubMed] [Google Scholar]
- 25.Boogerd LSF, Handgraaf HJM, Huurman VAL, et al. The Best Approach for Laparoscopic Fluorescence Cholangiography: Overview of the Literature and Optimization of Dose and Dosing Time. Surg Innov 2017;24:386-96. 10.1177/1553350617702311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata H, Aoki T, Koizumi T, et al. The Efficacy of Intraoperative Fluorescent Imaging Using Indocyanine Green for Cholangiography During Cholecystectomy and Hepatectomy. Clin Exp Gastroenterol 2021;14:145-54. 10.2147/CEG.S275985 [DOI] [PMC free article] [PubMed] [Google Scholar]