Abstract
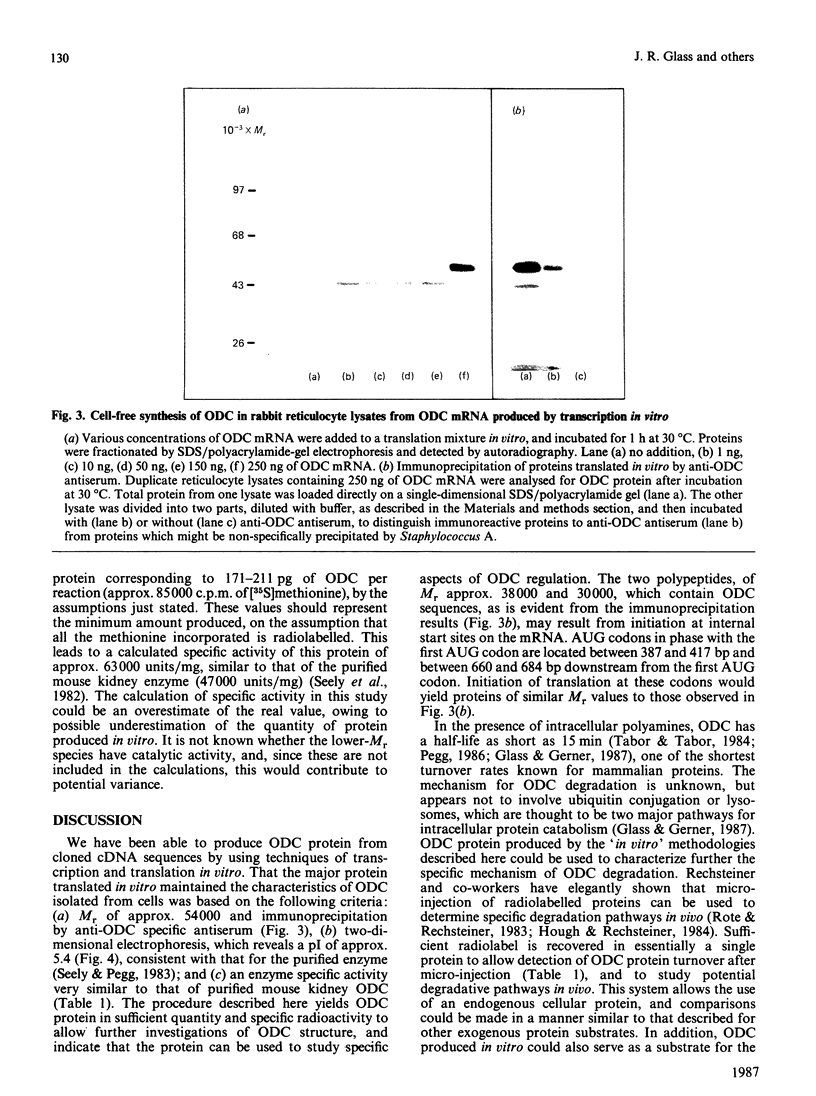
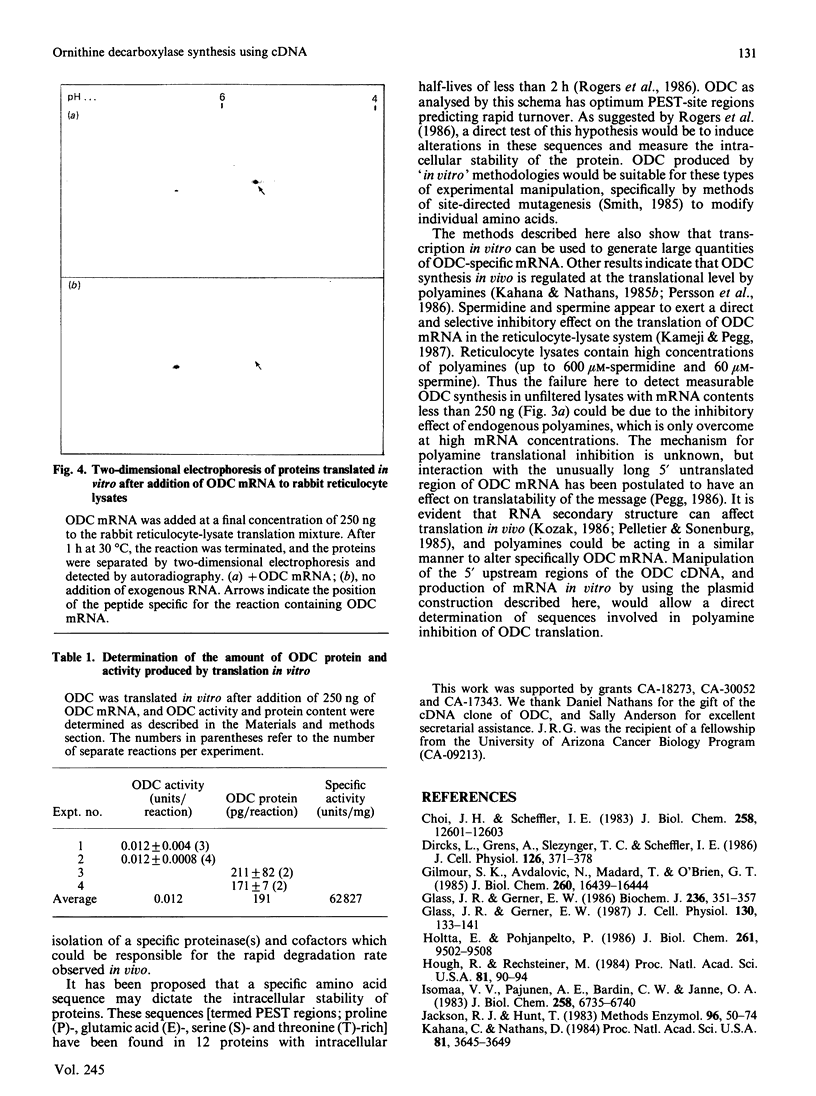
Microgram quantities of ornithine decarboxylase (ODC, EC 4.1.1.17)-specific mRNA were synthesized by transcription techniques in vitro, by using a plasmid containing mouse cDNA coding for this enzyme. The homogeneous RNA preparation was then used for cell-free synthesis of ODC protein, in rabbit reticulocyte lysates. Analysis of products translated in vitro by polyacrylamide-gel electrophoresis revealed predominantly one protein produced, with Mr approx. 54,000, which was immunoprecipitable by anti-ODC serum. Two-dimensional gel-electrophoretic analysis showed that the protein ODC synthesized in vitro had a pI of approx. 5.4, similar to the native enzyme isolated from mouse tissues. In addition, quantification of activity and protein amount showed that the enzyme synthesized in vitro had a specific activity of approx. 63,000 units (nmol/min)/mg, consistent with the purified mouse kidney enzyme's specific activity of approx. 47,000 units/mg. An average of nearly 200 pg of ODC protein was produced in vitro from various RNA preparations. These data demonstrate that ODC-specific mRNA and active ODC protein can be produced by 'in vitro' technology, which should prove useful in studying functional and structural characteristics of these molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi J. H., Scheffler I. E. Chinese hamster ovary cells resistant to alpha-difluoromethylornithine are overproducers of ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12601–12608. [PubMed] [Google Scholar]
- Dircks L., Grens A., Slezynger T. C., Scheffler I. E. Posttranscriptional regulation of ornithine decarboxylase activity. J Cell Physiol. 1986 Mar;126(3):371–378. doi: 10.1002/jcp.1041260307. [DOI] [PubMed] [Google Scholar]
- Gilmour S. K., Avdalovic N., Madara T., O'Brien T. G. Induction of ornithine decarboxylase by 12-O-tetradecanoylphorbol 13-acetate in hamster fibroblasts. Relationship between levels of enzyme activity, immunoreactive protein, and RNA during the induction process. J Biol Chem. 1985 Dec 25;260(30):16439–16444. [PubMed] [Google Scholar]
- Glass J. R., Gerner E. W. Polyamine-mediated turnover of ornithine decarboxylase in Chinese-hamster ovary cells. Biochem J. 1986 Jun 1;236(2):351–357. doi: 10.1042/bj2360351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J. R., Gerner E. W. Spermidine mediates degradation of ornithine decarboxylase by a non-lysosomal, ubiquitin-independent mechanism. J Cell Physiol. 1987 Jan;130(1):133–141. doi: 10.1002/jcp.1041300119. [DOI] [PubMed] [Google Scholar]
- Hough R., Rechsteiner M. Effects of temperature on the degradation of proteins in rabbit reticulocyte lysates and after injection into HeLa cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):90–94. doi: 10.1073/pnas.81.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Nucleotide sequence of murine ornithine decarboxylase mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1673–1677. doi: 10.1073/pnas.82.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Kanamoto R., Utsunomiya K., Kameji T., Hayashi S. Effects of putrescine on synthesis and degradation of ornithine decarboxylase in primary cultured hepatocytes. Eur J Biochem. 1986 Feb 3;154(3):539–544. doi: 10.1111/j.1432-1033.1986.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Coffino P. A mouse lymphoma cell mutant whose major protein product is ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12083–12086. [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986 Sep 15;205(2):175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Seely J. E., Persson L., Sertich G. J., Pegg A. E. Comparison of ornithine decarboxylase from rat liver, rat hepatoma and mouse kidney. Biochem J. 1985 Mar 1;226(2):577–586. doi: 10.1042/bj2260577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Smith M. In vitro mutagenesis. Annu Rev Genet. 1985;19:423–462. doi: 10.1146/annurev.ge.19.120185.002231. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]