Abstract
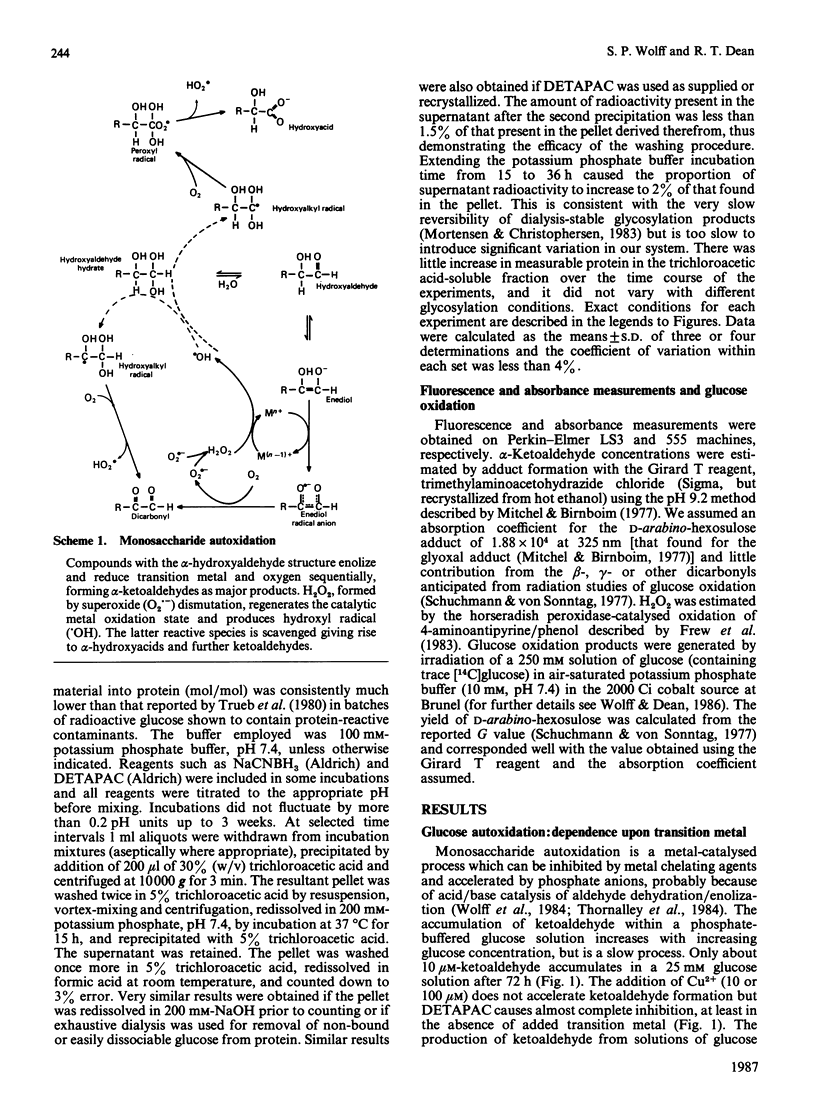
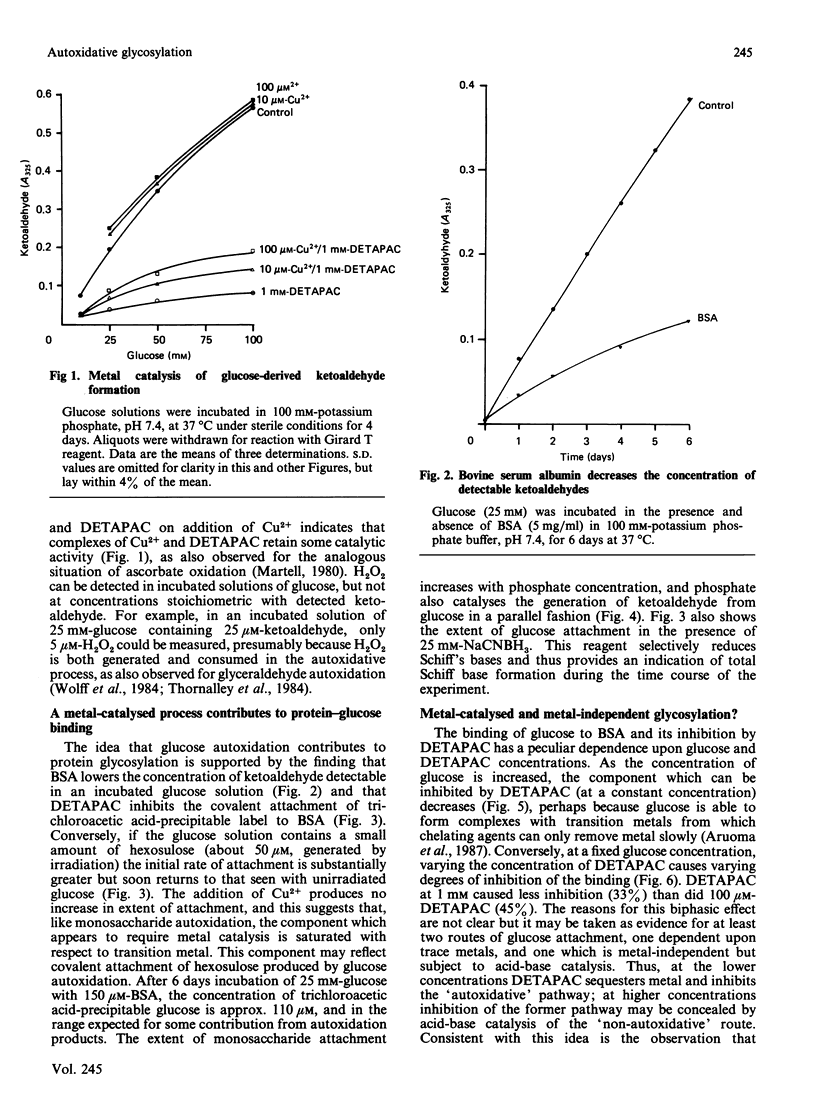
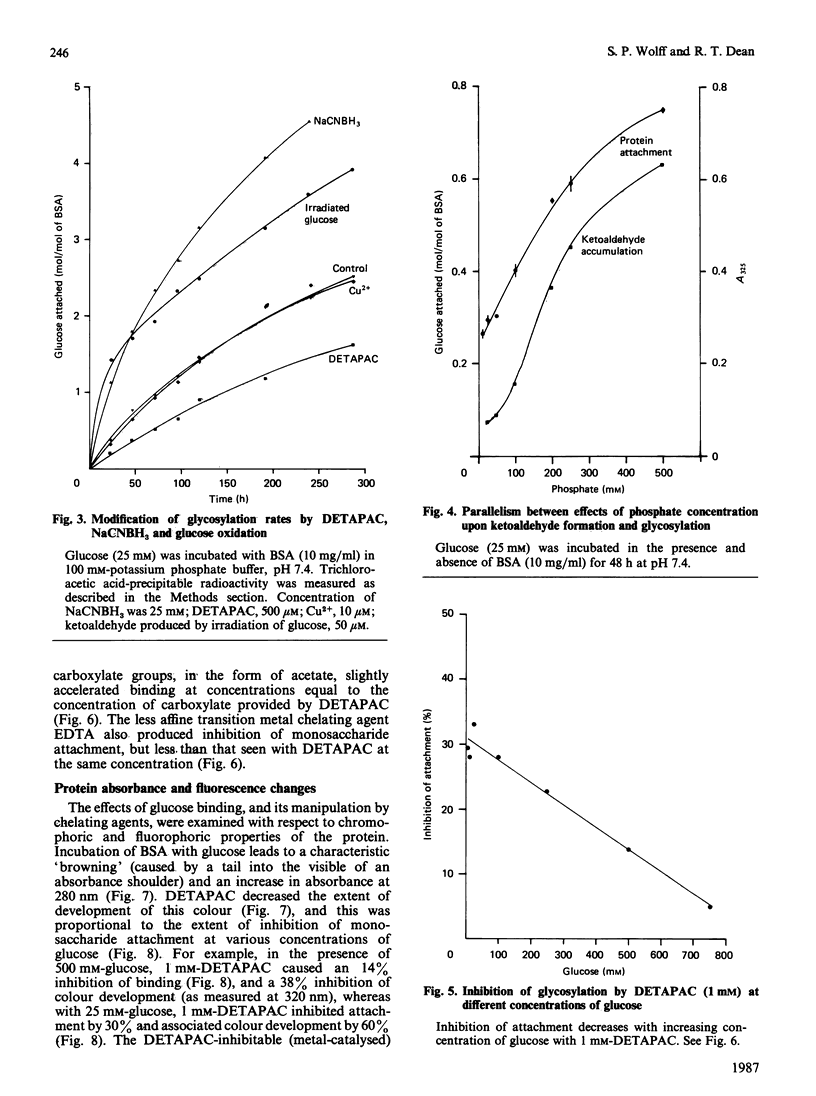
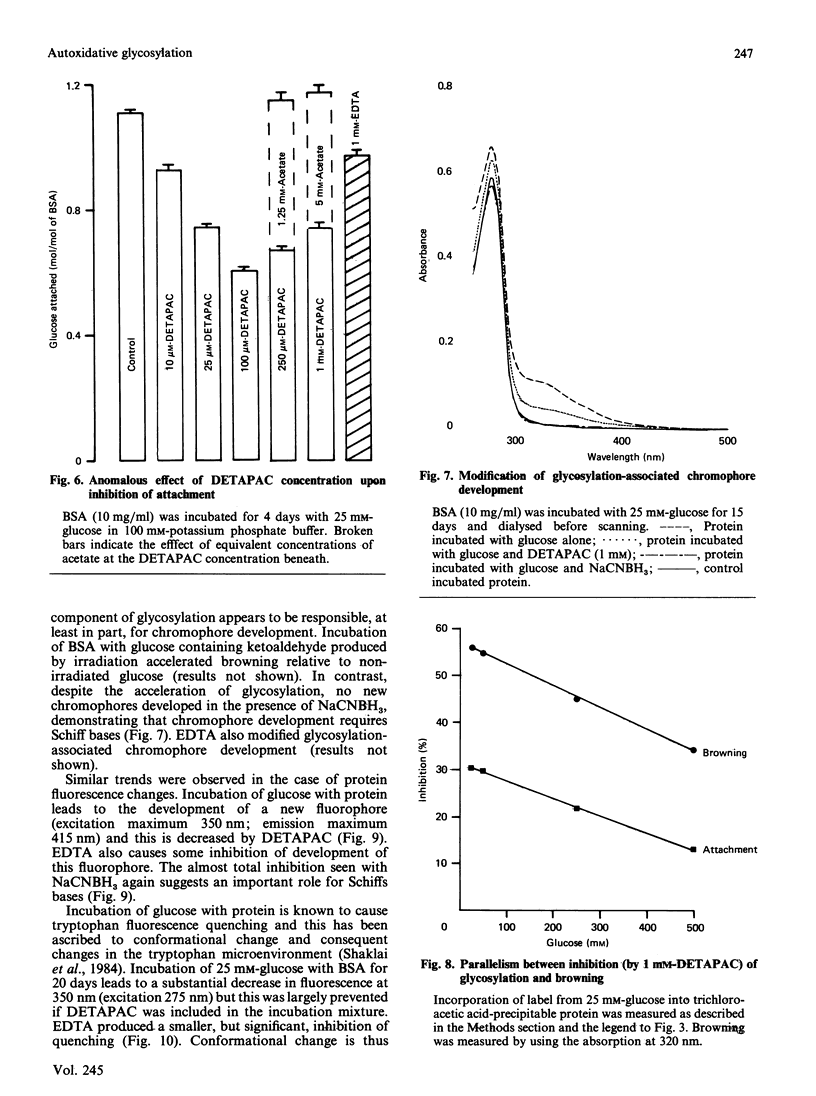
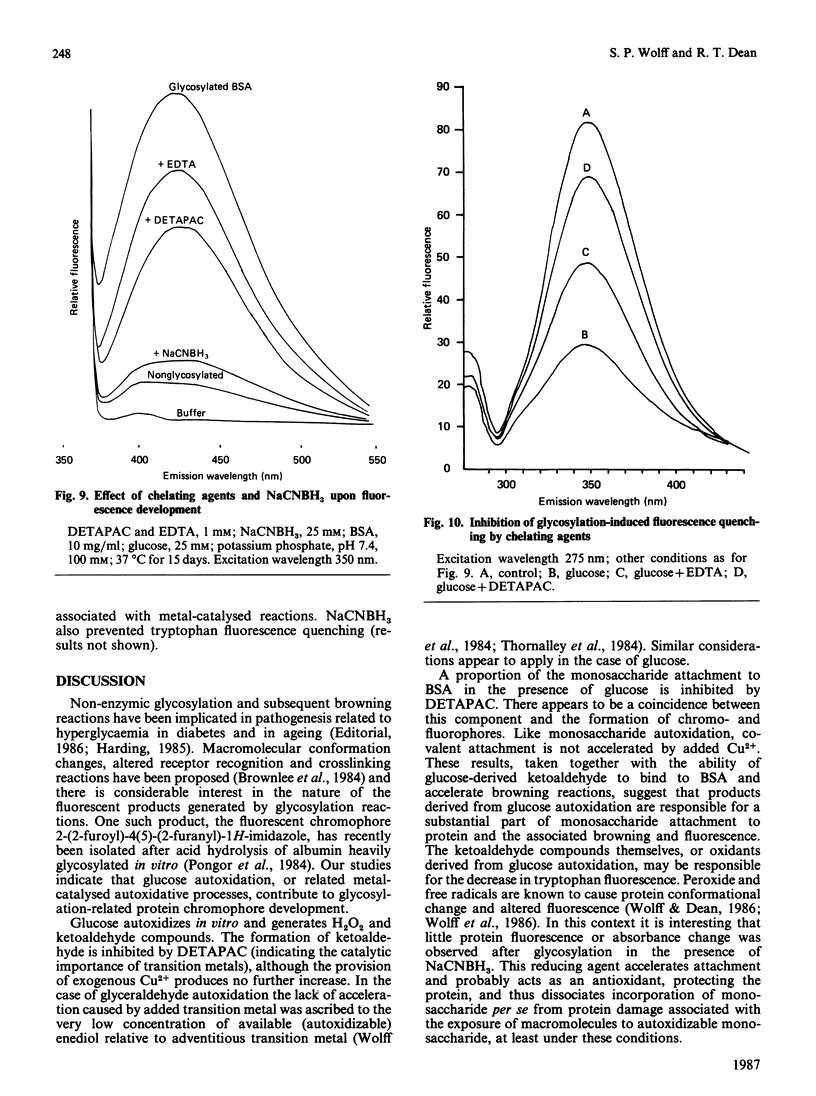
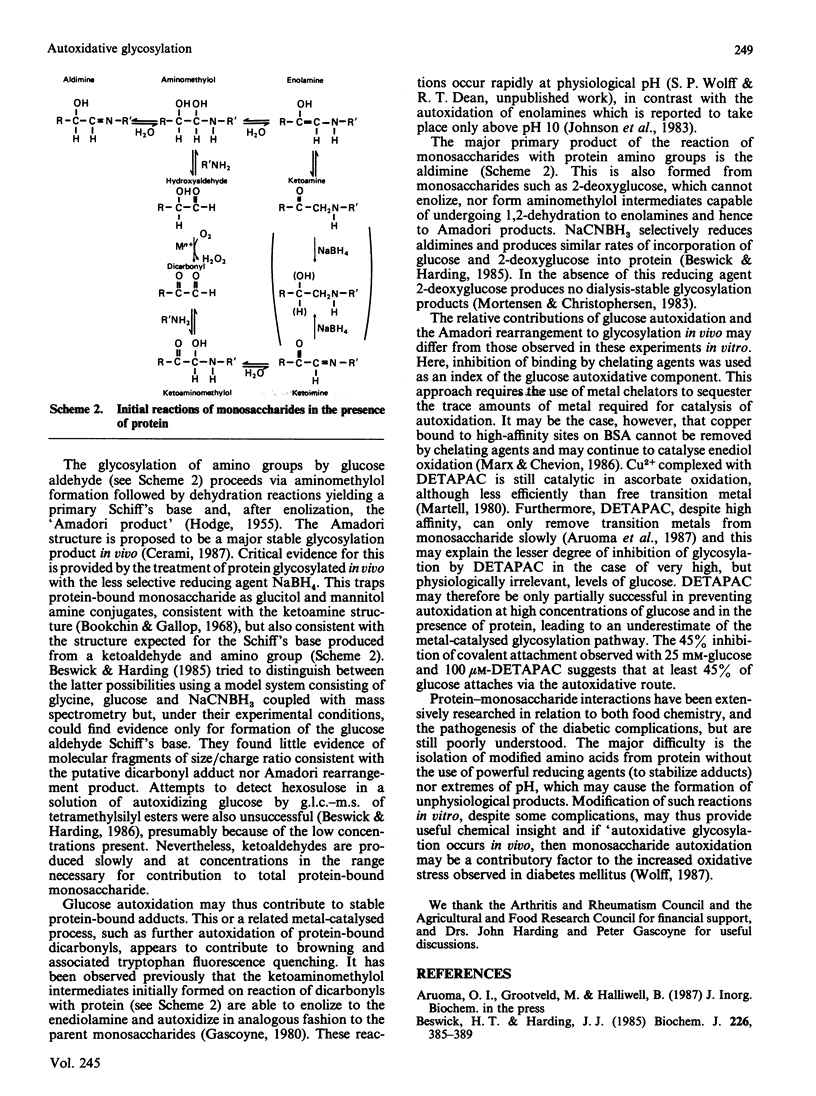
Monosaccharide autoxidation (a transition metal-catalysed process that generates H2O2 and ketoaldehydes) appears to contribute to protein modification by glucose in vitro. The metal-chelating agent diethylenetriaminepenta-acetic acid (DETAPAC), which inhibits glucose autoxidation, also reduces the covalent attachment of glucose to bovine serum albumin. A maximal 45% inhibition of covalent attachment was observed, but this varied with glucose and DETAPAC concentrations in a complex fashion, suggesting at least two modes of attachment. The extent of inhibition of the metal-catalysed pathway correlated with the extent of inhibition of glycosylation-associated chromo- and fluorophore development. DETAPAC also inhibited tryptophan fluorescence quenching associated with glycosylation. Conversely, ketoaldehydes analogous to those produced by glucose autoxidation, but generated by 60Co irradiation, bound avidly to albumin and accelerated browning reactions. It is therefore suggested that a component of protein glycosylation is dependent upon glucose autoxidation and subsequent covalent attachment of ketoaldehydes. The process of glucose autoxidation, or ketoaldehydes derived therefrom, appear to be important in chromophoric and fluorophoric alterations. It is noted, consistent with these observations, that the chemical evidence for the currently accepted 'Amadori' product derived from the reaction of glucose with protein amino groups is consistent also with the structure expected for the attachment of a glucose-derived ketoaldehyde to protein. The concept of 'autoxidative glycosylation' is briefly discussed in relation to oxidative stress in diabetes mellitus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beswick H. T., Harding J. J. Aldehydes or dicarbonyls in non-enzymic glycosylation of proteins. Biochem J. 1985 Mar 1;226(2):385–389. doi: 10.1042/bj2260385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Cogan D. G., Kinoshita J. H., Kador P. F., Robison G., Datilis M. B., Cobo L. M., Kupfer C. NIH conference. Aldose reductase and complications of diabetes. Ann Intern Med. 1984 Jul;101(1):82–91. doi: 10.7326/0003-4819-101-1-82. [DOI] [PubMed] [Google Scholar]
- HODGE J. E. The Amadori rearrangement. Adv Carbohydr Chem. 1955;10:169–205. doi: 10.1016/s0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv Protein Chem. 1985;37:247–334. doi: 10.1016/s0065-3233(08)60066-2. [DOI] [PubMed] [Google Scholar]
- Johnson R. N., Metcalf P. A., Baker J. R. Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta. 1983 Jan 7;127(1):87–95. doi: 10.1016/0009-8981(83)90078-5. [DOI] [PubMed] [Google Scholar]
- Marx G., Chevion M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem J. 1986 Jun 1;236(2):397–400. doi: 10.1042/bj2360397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. A., Pethig R., Szent-Györgyi A. Spectroscopic studies of the protein-methylglyoxal adduct. Proc Natl Acad Sci U S A. 1980 Feb;77(2):949–951. doi: 10.1073/pnas.77.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel R. E., Birnboim H. C. The use of Girard-T reagent in a rapid and sensitive methods for measuring glyoxal and certain other alpha-dicarbonyl compounds. Anal Biochem. 1977 Jul;81(1):47–56. doi: 10.1016/0003-2697(77)90597-8. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Vishwanath V., Frank K. E., Elmets C. A., Dauchot P., Kohn R. R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986 Feb 13;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- Mortensen H. B., Christophersen C. Glucosylation of human haemoglobin a in red blood cells studied in vitro. Kinetics of the formation and dissociation of haemoglobin A1c. Clin Chim Acta. 1983 Nov 15;134(3):317–326. doi: 10.1016/0009-8981(83)90370-4. [DOI] [PubMed] [Google Scholar]
- Nishigaki I., Hagihara M., Tsunekawa H., Maseki M., Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem Med. 1981 Jun;25(3):373–378. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- Pongor S., Ulrich P. C., Bencsath F. A., Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984 May;81(9):2684–2688. doi: 10.1073/pnas.81.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Hotta N., Sakamoto N., Matsuoka S., Ohishi N., Yagi K. Lipid peroxide level in plasma of diabetic patients. Biochem Med. 1979 Feb;21(1):104–107. doi: 10.1016/0006-2944(79)90061-9. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Garlick R. L., Bunn H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984 Mar 25;259(6):3812–3817. [PubMed] [Google Scholar]
- Thornalley P., Wolff S., Crabbe J., Stern A. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984 Feb 14;797(2):276–287. doi: 10.1016/0304-4165(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Holenstein C. G., Fischer R. W., Winterhalter K. H. Nonenzymatic glycosylation of proteins. A warning. J Biol Chem. 1980 Jul 25;255(14):6717–6720. [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]


