Abstract
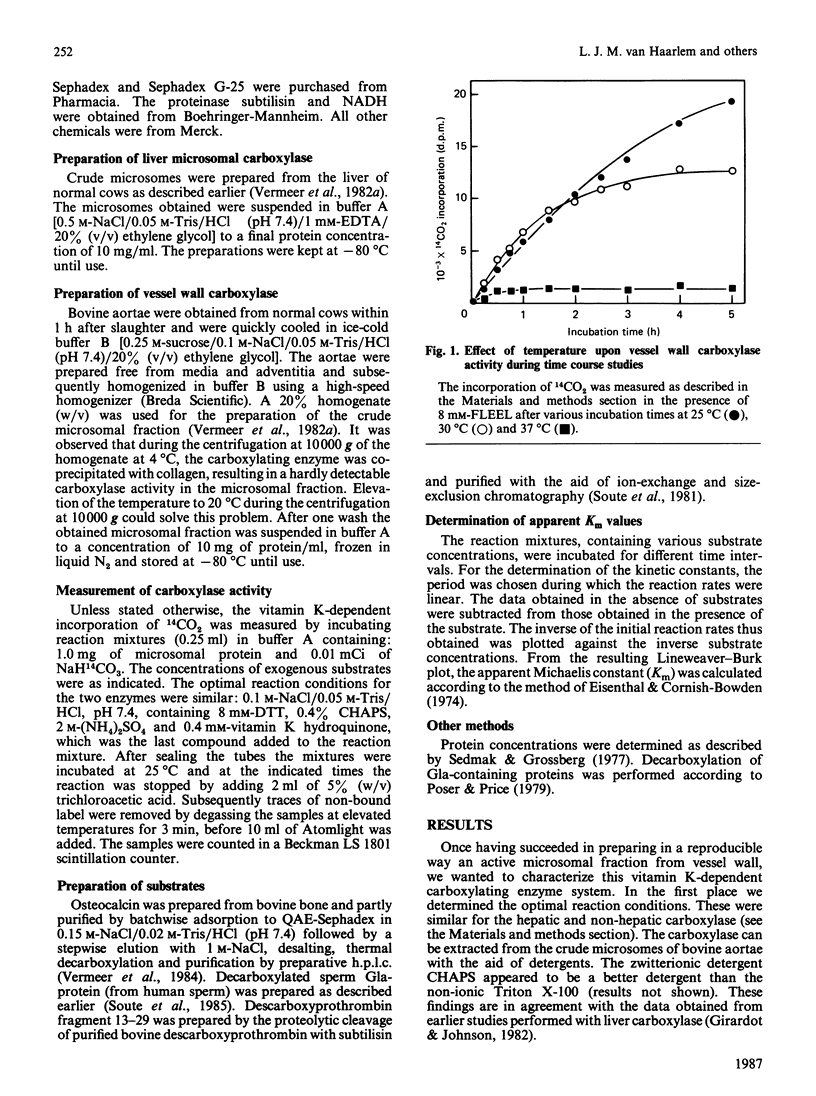
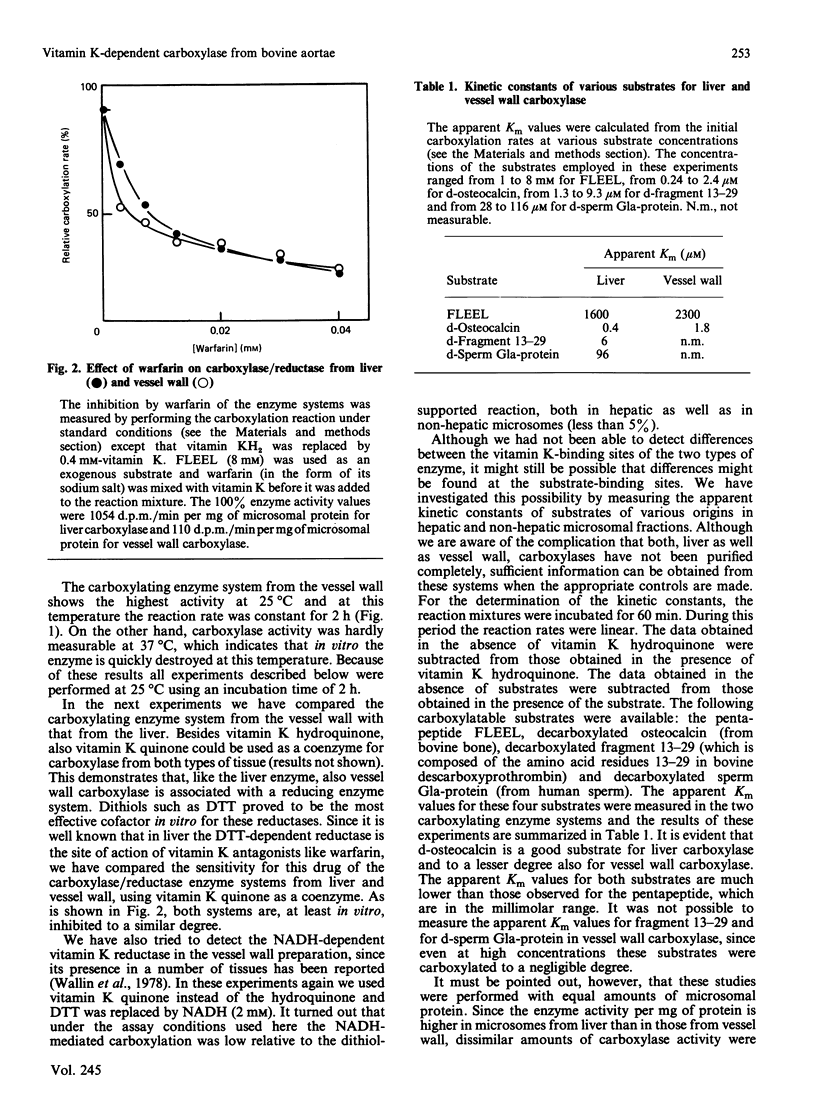
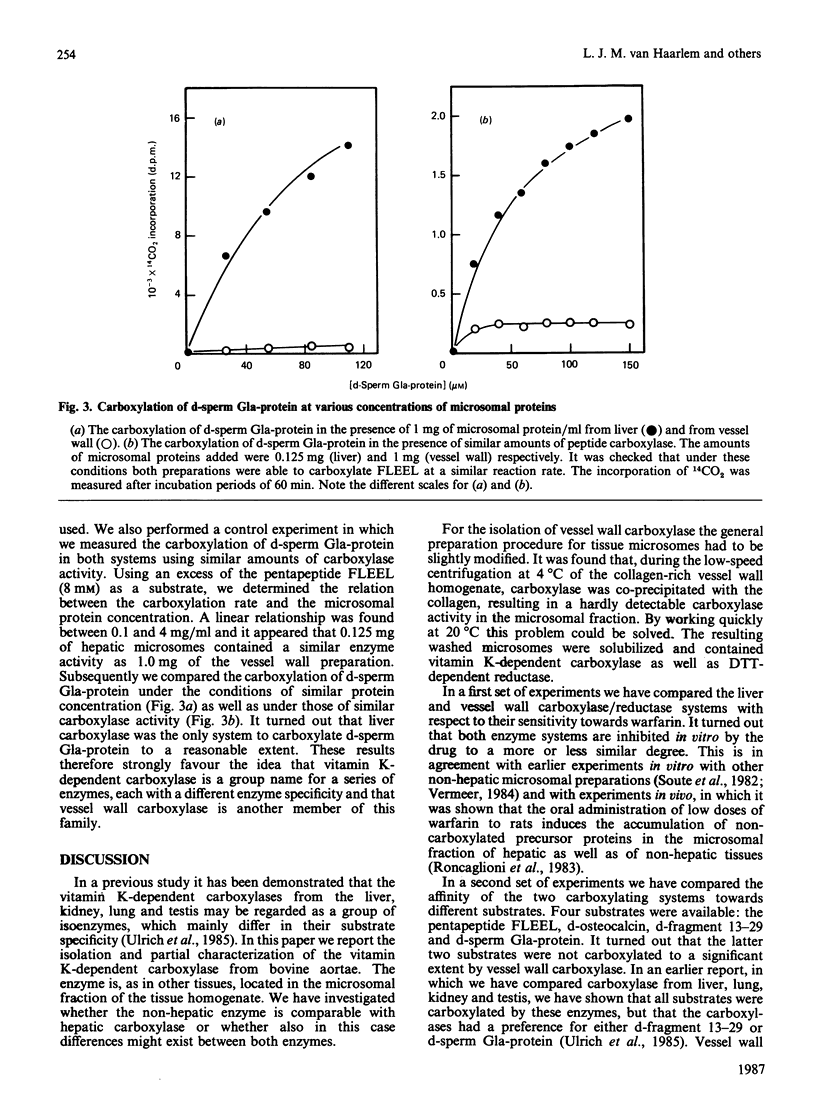
Vitamin K-dependent carboxylase activity has been demonstrated in the crude microsomal fraction of the intima of bovine aortae. The procedure for the isolation of vessel wall carboxylase is a slight modification of the general preparation procedure for tissue microsomes. The highest activity of the non-hepatic enzyme was observed at 25 degrees C and hardly any NADH-dependent vitamin K reductase could be demonstrated. The optimal reaction conditions for both vessel wall as well as liver carboxylase were similar: 0.1 M-NaCl/0.05 M-Tris/HCl, pH 7.4, containing 8 mM-dithiothreitol, 0.4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonic acid (CHAPS), 0.4 mM-vitamin K hydroquinone and 2 M-(NH4)2SO4. Warfarin inhibits the hepatic and non-hepatic carboxylase/reductase enzyme complex more or less to a similar degree. We have measured the apparent Km values for the following substrates: Phe-Leu-Glu-Glu-Leu ('FLEEL'), decarboxylated osteocalcin, decarboxylated fragment 13-29 from descarboxyprothrombin and decarboxylated sperm 4-carboxyglutamic acid-containing (Gla-)protein. The results obtained demonstrated that liver and vessel wall carboxylase may be regarded as isoenzymes with different substrate specificities. The newly discovered enzyme is the first vitamin K-dependent carboxylase which shows an absolute substrate specificity: FLEEL and decarboxylated osteocalcin were good substrates for vessel wall carboxylase, but decarboxylated fragment 13-29 and decarboxylated sperm Gla-protein were not carboxylated at all.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchthal S. D., Bell R. G. Vitamin K dependent carboxylation of glutamate residues to gamma-carboxyglutamate in microsomes from spleen and testes: comparison with liver, lung, and kidney. Biochemistry. 1983 Mar 1;22(5):1077–1082. doi: 10.1021/bi00274a013. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Sadowski J. A., Suttie J. W. A new carboxylation reaction. The vitamin K-dependent incorporation of H-14-CO3- into prothrombin. J Biol Chem. 1975 Jun 25;250(12):4744–4748. [PubMed] [Google Scholar]
- Girardot J. M., Johnson B. C. A new detergent for the solubilization of the vitamin K-dependent carboxylation system from liver microsomes: comparison with triton X-100. Anal Biochem. 1982 Apr;121(2):315–320. doi: 10.1016/0003-2697(82)90486-9. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. J., Howard S. L., Oshry L. J. Carboxyglutamic acid (Gla) containing proteins of human calcified atherosclerotic plaque solubilized by EDTA. Molecular weight distribution and relationship to osteocalcin. Atherosclerosis. 1986 Feb;59(2):155–160. doi: 10.1016/0021-9150(86)90044-4. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Lian J. B., Gallop P. Atherocalcin, a gamma-carboxyglutamic acid containing protein from atherosclerotic plaque. Biochem Biophys Res Commun. 1979 Nov 14;91(1):41–49. doi: 10.1016/0006-291x(79)90580-1. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Prien E. L., Jr, Glimcher M. J., Gallop P. M. The presence of protein-bound gamma-carboxyglutamic acid in calcium-containing renal calculi. J Clin Invest. 1977 Jun;59(6):1151–1157. doi: 10.1172/JCI108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Roncaglioni M. C., Soute B. A., de Boer-vd Berg M. A., Vermeer C. Warfarin-induced accumulation of vitamin K-dependent proteins. Comparison between hepatic and non-hepatic tissues. Biochem Biophys Res Commun. 1983 Aug 12;114(3):991–997. doi: 10.1016/0006-291x(83)90658-7. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Soute B. A., Müller-Esterl W., de Boer-van den Berg M. A., Ulrich M., Vermeer C. Discovery of a gamma-carboxyglutamic acid-containing protein in human spermatozoa. FEBS Lett. 1985 Oct 7;190(1):137–141. doi: 10.1016/0014-5793(85)80444-0. [DOI] [PubMed] [Google Scholar]
- Soute B. A., de Metz M., Vermeer C. Characteristics of vitamin K-dependent carboxylating systems from human liver and placenta. FEBS Lett. 1982 Sep 20;146(2):365–368. doi: 10.1016/0014-5793(82)80954-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Lehrman S. R., Geweke L. O., Hageman J. M., Rich D. H. Vitamin K-dependent carboxylase: requirements for carboxylation of soluble peptide and substrate specificity. Biochem Biophys Res Commun. 1979 Feb 14;86(3):500–507. doi: 10.1016/0006-291x(79)91742-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Mechanism of action of vitamin K: synthesis of gamma-carboxyglutamic acid. CRC Crit Rev Biochem. 1980;8(2):191–223. doi: 10.3109/10409238009105469. [DOI] [PubMed] [Google Scholar]
- Ulrich M. M., Soute B. A., de Boer-van den Berg M. A., Vermeer C. Isoenzymes of vitamin-K-dependent carboxylase. Biochim Biophys Acta. 1985 Jul 18;830(1):105–108. doi: 10.1016/0167-4838(85)90138-4. [DOI] [PubMed] [Google Scholar]
- Vermeer C., Hendrix H., Daemen M. Vitamin K-dependent carboxylases from non-hepatic tissues. FEBS Lett. 1982 Nov 8;148(2):317–320. doi: 10.1016/0014-5793(82)80832-6. [DOI] [PubMed] [Google Scholar]
- Vermeer C., Soute B. A., De Metz M., Hemker H. C. A comparison between vitamin K-dependent carboxylase from normal and warfarin-treated cows. Biochim Biophys Acta. 1982 Feb 2;714(2):361–365. doi: 10.1016/0304-4165(82)90346-4. [DOI] [PubMed] [Google Scholar]
- Vermeer C., Soute B. A., Hendrix H., de Boer-van den Berg M. A. Decarboxylated bone Gla-protein as a substrate for hepatic vitamin K-dependent carboxylase. FEBS Lett. 1984 Jan 2;165(1):16–20. doi: 10.1016/0014-5793(84)80005-8. [DOI] [PubMed] [Google Scholar]
- Vermeer C. The vitamin K-dependent carboxylation reaction. Mol Cell Biochem. 1984;61(1):17–35. doi: 10.1007/BF00239604. [DOI] [PubMed] [Google Scholar]
- Wallin R., Gebhardt O., Prydz H. NAD(P)H dehydrogenase and its role in the vitamin K (2-methyl-3-phytyl-1,4-naphthaquinone)-dependent carboxylation reaction. Biochem J. 1978 Jan 1;169(1):95–101. doi: 10.1042/bj1690095. [DOI] [PMC free article] [PubMed] [Google Scholar]


