Abstract
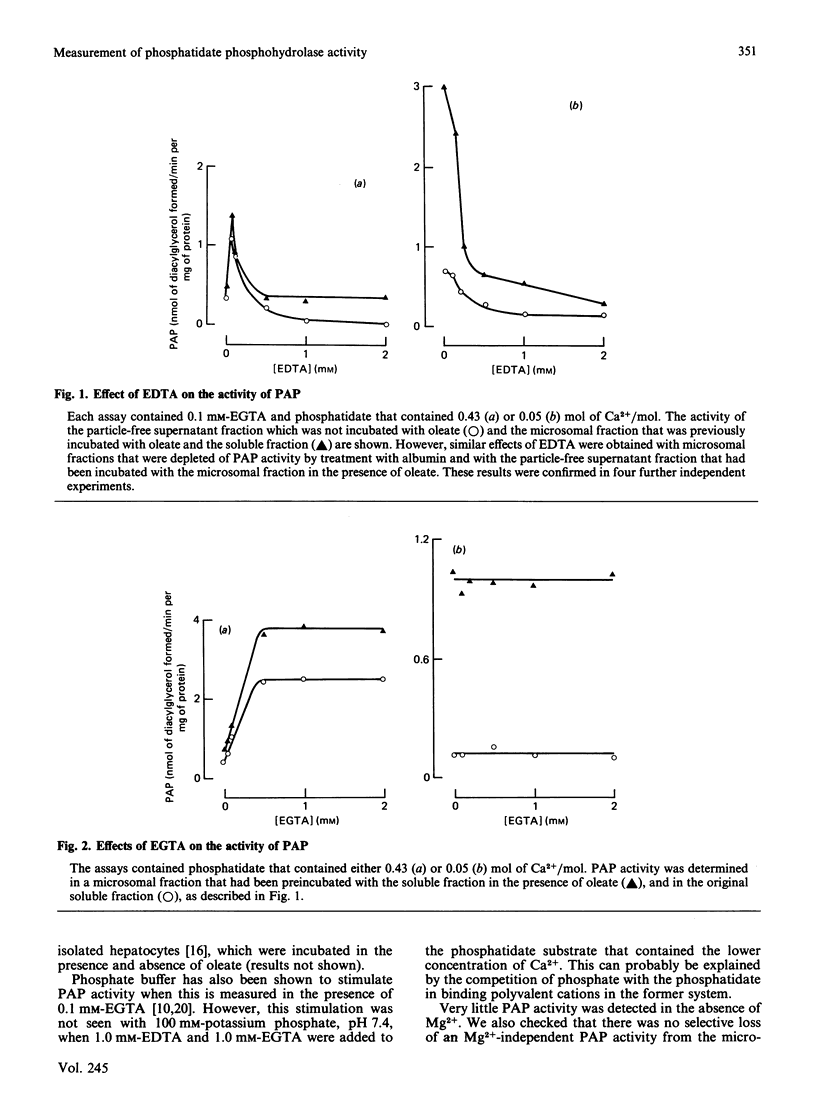
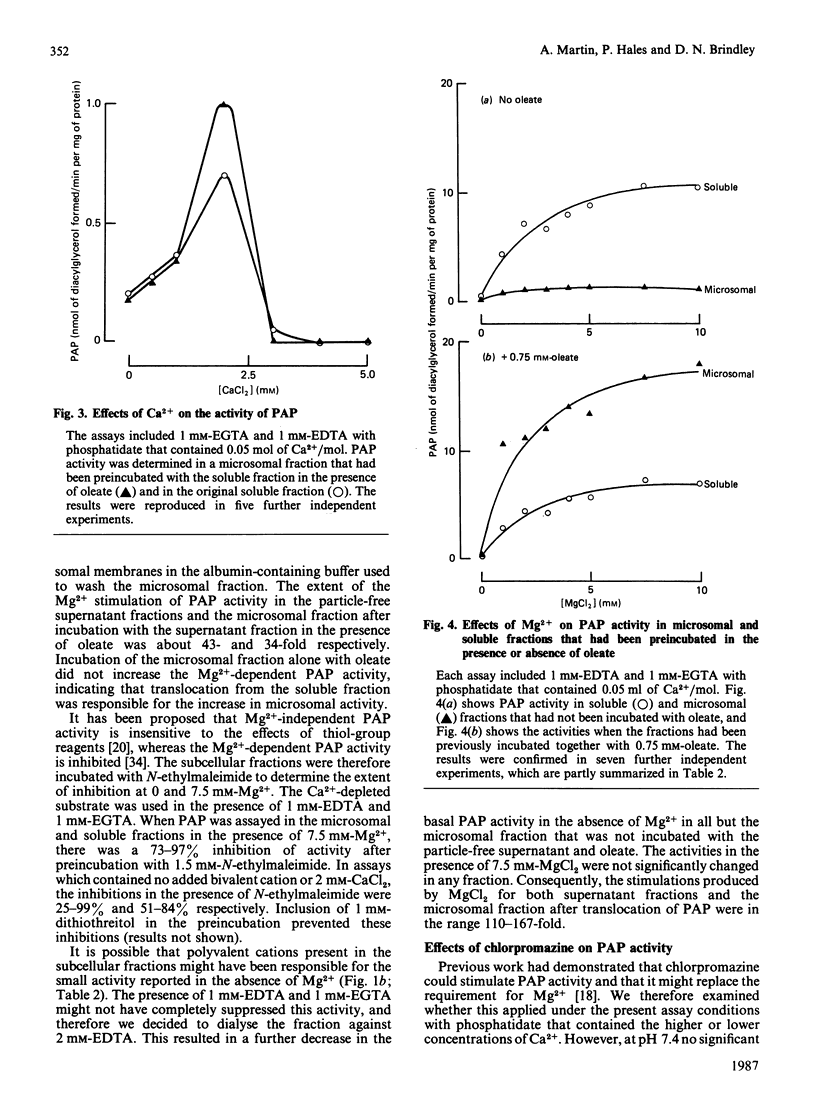
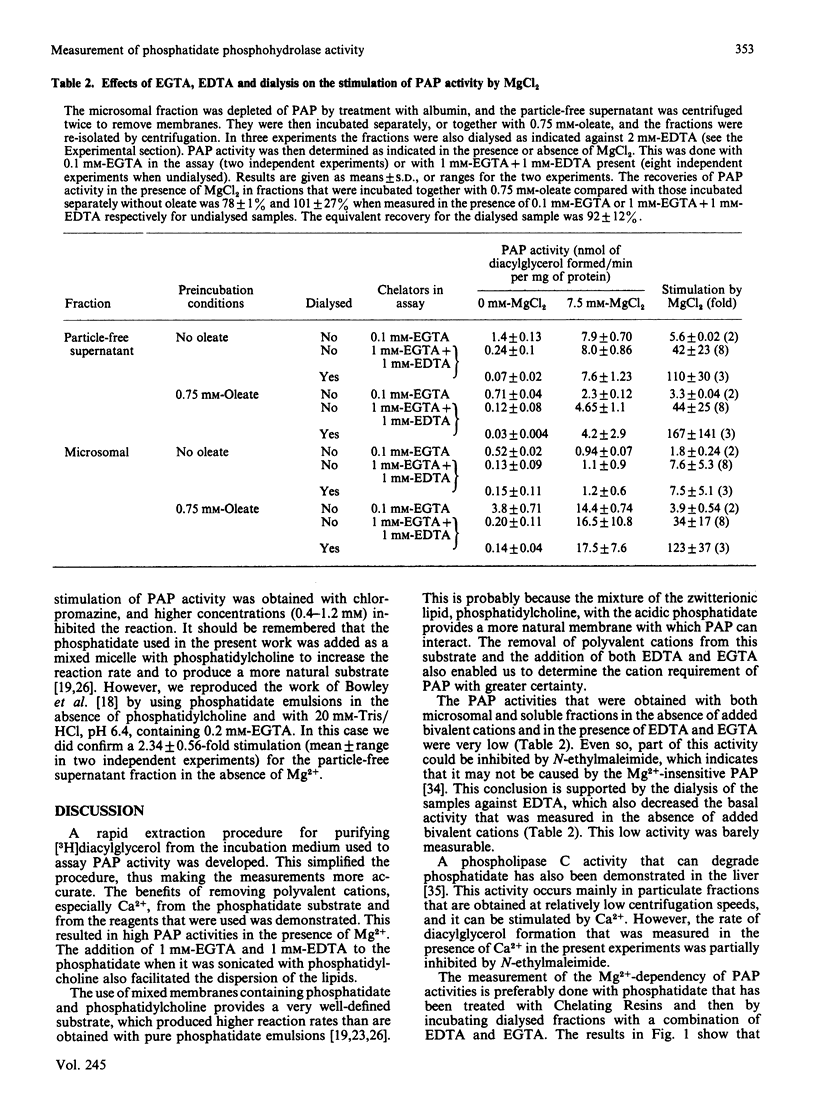
1. A rapid extraction and purification scheme was designed for the recovery of [3H]diacylglycerol formed during the assay of phosphatidate phosphohydrolase. 2. The importance of removing polyvalent cations, particularly Ca2+, from the phosphatidate and other reagents used in the assay of the phosphohydrolase activity was demonstrated. This was achieved mainly by treating the phosphatidate with a chelating resin and by adding 1 mM-EGTA and 1 mM-EDTA to the assays. 3. The activity of the phosphohydrolase in dialysed samples of the soluble and microsomal fractions of rat liver was very low. 4. Addition of optimum concentrations of MgCl2 resulted in a 110-167-fold stimulation in activity. 5. CaCl2 was also able to stimulate phosphohydrolase activity, but to a much smaller extent than MgCl2. 6. Chlorpromazine, an amphiphilic cation, inhibited the reaction when it was measured in these experiments by using a mixed emulsion of phosphatidylcholine and phosphatidate at pH 7.4. 7. Microsomal fractions that were preincubated with albumin contained very low activities of the Mg2+-dependent phosphohydrolase. When these were then incubated with the soluble fraction in the presence of oleate, the soluble phosphohydrolase attached to the microsomal membranes, and it retained its high dependency on Mg2+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowley M., Cooling J., Burditt S. L., Brindley D. N. The effects of amphiphilic cationic drugs and inorganic cations on the activity of phosphatidate phosphohydrolase. Biochem J. 1977 Sep 1;165(3):447–454. doi: 10.1042/bj1650447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley D. N. Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res. 1984;23(3):115–133. doi: 10.1016/0163-7827(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Butterwith S. C., Hopewell R., Brindley D. N. Partial purification and characterization of the soluble phosphatidate phosphohydrolase of rat liver. Biochem J. 1984 Jun 15;220(3):825–833. doi: 10.1042/bj2200825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwith S. C., Martin A., Brindley D. N. Can phosphorylation of phosphatidate phosphohydrolase by a cyclic AMP-dependent mechanism regulate its activity and subcellular distribution and control hepatic glycerolipid synthesis? Biochem J. 1984 Sep 1;222(2):487–493. doi: 10.1042/bj2220487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales C., Mangiapane E. H., Brindley D. N. Oleic acid promotes the activation and translocation of phosphatidate phosphohydrolase from the cytosol to particulate fractions of isolated rat hepatocytes. Biochem J. 1984 May 1;219(3):911–916. doi: 10.1042/bj2190911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hopewell R., Martin-Sanz P., Martin A., Saxton J., Brindley D. N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem J. 1985 Dec 1;232(2):485–491. doi: 10.1042/bj2320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S., Numa S. Partial purification, properties, and subcellulsr distribution of rat liver phosphatidate phosphatase. J Biochem. 1975 Mar;77(3):501–509. doi: 10.1093/oxfordjournals.jbchem.a130751. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J. Glycerolipid biosynthesis in rat adipose tissue. 11. Effects of polyamines on Mg2+-dependent phosphatidate phosphohydrolase. Biochim Biophys Acta. 1983 Jun 16;752(1):79–88. doi: 10.1016/0005-2760(83)90235-7. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J., Wells G. N. Glycerolipid biosynthesis in rat adipose tissue 12. Properties of Mg2+-dependent and -independent phosphatidate phosphohydrolase. Arch Biochem Biophys. 1984 Sep;233(2):370–377. doi: 10.1016/0003-9861(84)90458-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. G., Schwertz D. W. The effects of bromobenzene and carbon tetrachloride exposure in vitro on the phospholipase C activity of rat liver cells. Toxicol Appl Pharmacol. 1982 Apr;63(2):216–229. doi: 10.1016/0041-008x(82)90044-8. [DOI] [PubMed] [Google Scholar]
- Lawson N., Pollard A. D., Jennings R. J., Gurr M. I., Brindley D. N. The activities of lipoprotein lipase and of enzymes involved in triacylglycerol synthesis in rat adipose tissue. Effects of starvation, dietary modification and of corticotropin injection. Biochem J. 1981 Nov 15;200(2):285–294. doi: 10.1042/bj2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanz P., Hopewell R., Brindley D. N. Spermine promotes the translocation of phosphatidate phosphohydrolase from the cytosol to the microsomal fraction of rat liver and it enhances the effects of oleate in this respect. FEBS Lett. 1985 Jan 7;179(2):262–266. doi: 10.1016/0014-5793(85)80531-7. [DOI] [PubMed] [Google Scholar]
- Martin A., Hopewell R., Martín-Sanz P., Morgan J. E., Brindley D. N. Relationship between the displacement of phosphatidate phosphohydrolase from the membrane-associated compartment by chlorpromazine and the inhibition of the synthesis of triacylglycerol and phosphatidylcholine in rat hepatocytes. Biochim Biophys Acta. 1986 May 21;876(3):581–591. doi: 10.1016/0005-2760(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Moller F., Hough M. R. Effect of salts on membrane binding and activity of adipocyte phosphatidate phosphohydrolase. Biochim Biophys Acta. 1982 Jun 11;711(3):521–531. doi: 10.1016/0005-2760(82)90068-6. [DOI] [PubMed] [Google Scholar]
- Moller F., Wong K. H., Green P. Control of fat cell phosphohydrolase by lipolytic agents. Can J Biochem. 1981 Jan;59(1):9–15. doi: 10.1139/o81-002. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Pritchard P. H., Brindley D. N., Vance D. E. Fatty acids reverse the cyclic AMP inhibition of triacylglycerol and phosphatidylcholine synthesis in rat hepatocytes. Biochem J. 1983 Oct 15;216(1):129–136. doi: 10.1042/bj2160129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Bracken P., Fears R., Brindley D. N. Insulin antagonises the growth hormone-mediated increase in the activity of phosphatidate phosphohydrolase in isolated rat hepatocytes. FEBS Lett. 1986 Jun 23;202(1):133–136. doi: 10.1016/0014-5793(86)80663-9. [DOI] [PubMed] [Google Scholar]
- Pittner R. A., Bracken P., Fears R., Brindley D. N. Spermine antagonises the effects of dexamethasone, glucagon and cyclic AMP in increasing the activity of phosphatidate phosphohydrolase in isolated rat hepatocytes. FEBS Lett. 1986 Oct 20;207(1):42–46. doi: 10.1016/0014-5793(86)80009-6. [DOI] [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Effects of cyclic AMP, glucocorticoids and insulin on the activities of phosphatidate phosphohydrolase, tyrosine aminotransferase and glycerol kinase in isolated rat hepatocytes in relation to the control of triacylglycerol synthesis and gluconeogenesis. Biochem J. 1985 Jan 15;225(2):455–462. doi: 10.1042/bj2250455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Effects of insulin, glucagon, dexamethasone, cyclic GMP and spermine on the stability of phosphatidate phosphohydrolase activity in cultured rat hepatocytes. Biochem J. 1986 Nov 15;240(1):253–257. doi: 10.1042/bj2400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Interactions of insulin, glucagon and dexamethasone in controlling the activity of glycerol phosphate acyltransferase and the activity and subcellular distribution of phosphatidate phosphohydrolase in cultured rat hepatocytes. Biochem J. 1985 Sep 1;230(2):525–534. doi: 10.1042/bj2300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen O. Mono- and dimethyl phosphatidates from different subtypes of choline and ethanolamine glycerophosphatides. Biochim Biophys Acta. 1968 Jan 10;152(1):114–135. doi: 10.1016/0005-2760(68)90014-3. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Factors controlling the activities of phosphatidate phosphohydrolase and phosphatidate cytidylyltransferase. The effects of chlorpromazine, demethylimipramine, cinchocaine, norfenfluramine, mepyramine and magnesium ions. Biochem J. 1977 Jan 15;162(1):25–32. doi: 10.1042/bj1620025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Factors controlling the metabolism of phosphatidate by phosphohydrolase and phospholipase A-type activities. Effects of magnesium, calcium and amphiphilic cationic drugs. Biochim Biophys Acta. 1980 Sep 8;619(3):494–505. doi: 10.1016/0005-2760(80)90101-0. [DOI] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Problems encountered in measuring the activity of phosphatidate phosphohydrolase. Biochem J. 1978 Apr 1;171(1):263–266. doi: 10.1042/bj1710263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. J., Saggerson E. D. Adipose-tissue Mg2+-dependent phosphatidate phosphohydrolase. Control of activity and subcellular distribution in vitro and in vivo. Biochem J. 1986 Oct 15;239(2):275–284. doi: 10.1042/bj2390275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Possmayer F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem. 1985 Dec;151(2):479–486. doi: 10.1016/0003-2697(85)90208-8. [DOI] [PubMed] [Google Scholar]


