Abstract
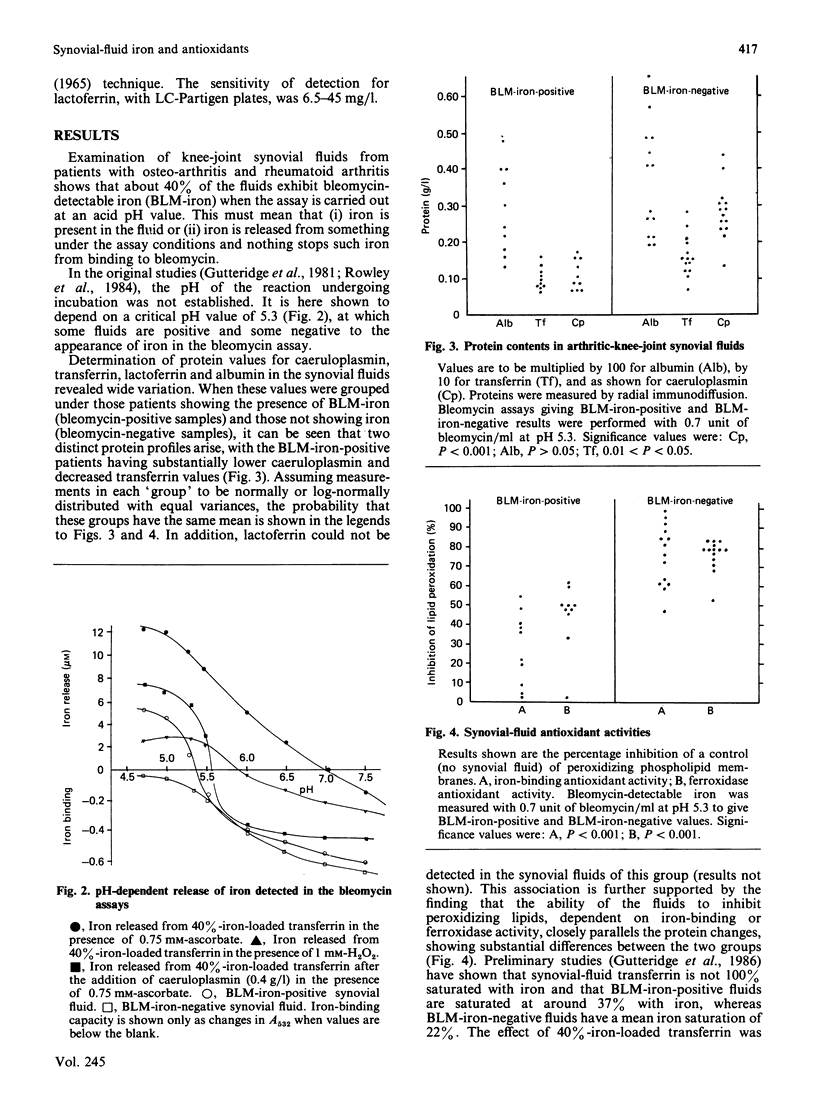
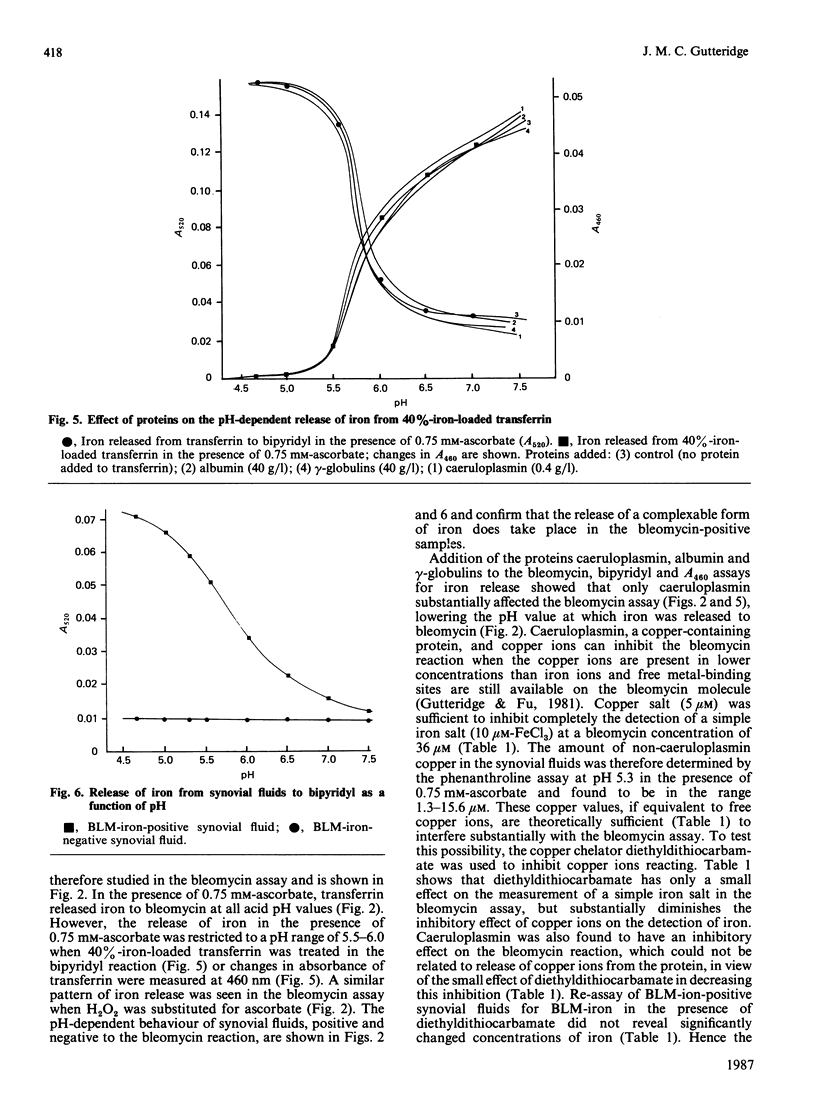
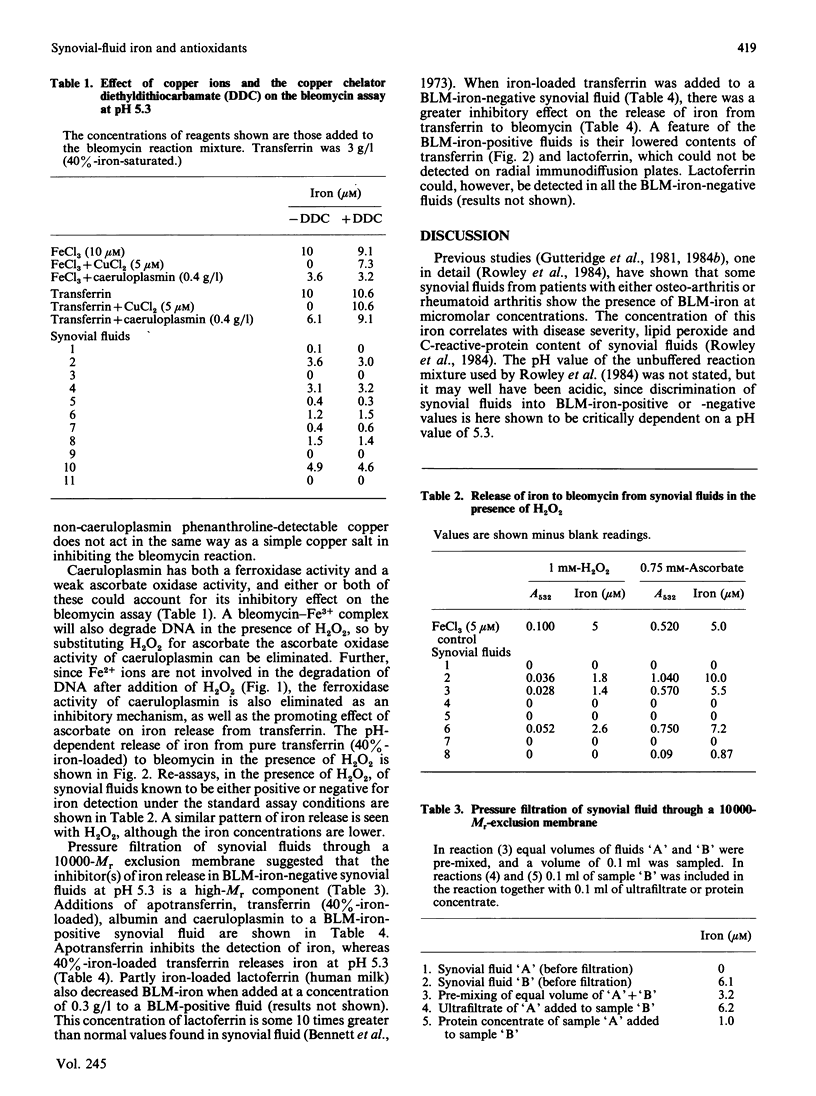
Some 40% of knee-joint synovial fluids from arthritic patients show the presence of bleomycin-detectable iron. This is released from a protein component of the fluid to bleomycin at acidic pH values. Patients whose fluids release iron have lower contents of transferrin, lactoferrin and caeruloplasmin than do patients whose fluids do not release iron to bleomycin. These proteins are important extracellular antioxidants, and measured antioxidant activities are extremely low in the iron-releasing fluids. The propensity of some fluids to release iron at low pH values, characteristic of the microenvironment beneath adherent macrophages, coupled with their decreased antioxidant protection against iron-stimulated oxygen-radical damage, might explain previously reported correlations between clinical disease severity, lipid peroxide content and the presence of bleomycin-detectable iron [Rowley, Gutteridge, Blake, Farr & Halliwell (1984) Clin. Sci. 66, 691-695].
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Eddie-Quartey A. C., Holt P. J. Lactoferrin--an iron binding protein in synovial fluid. Arthritis Rheum. 1973 Mar-Apr;16(2):186–190. doi: 10.1002/art.1780160208. [DOI] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Bacon P. A., Dieppe P. A., Halliwell B., Gutteridge J. M. The importance of iron in rheumatoid disease. Lancet. 1981 Nov 21;2(8256):1142–1144. doi: 10.1016/s0140-6736(81)90590-0. [DOI] [PubMed] [Google Scholar]
- Cranfield L. M., Gollan J. L., White A. G., Dormandy T. L. Serum antioxidant activity in normal and abnormal subjects. Ann Clin Biochem. 1979 Nov;16(6):299–306. doi: 10.1177/000456327901600181. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of the proteins caeruloplasmin, albumin and transferrin. A study of their activity in serum and synovial fluid from patients with rheumatoid arthritis. Biochim Biophys Acta. 1986 Jan 30;869(2):119–127. doi: 10.1016/0167-4838(86)90286-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J. 1984 Mar 15;218(3):983–985. doi: 10.1042/bj2180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B., Treffry A., Harrison P. M., Blake D. Effect of ferritin-containing fractions with different iron loading on lipid peroxidation. Biochem J. 1983 Feb 1;209(2):557–560. doi: 10.1042/bj2090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Hill C., Blake D. R. Copper stimulated phospholipid membrane peroxidation: antioxidant activity of serum and synovial fluid from patients with rheumatoid arthritis. Clin Chim Acta. 1984 May 16;139(1):85–90. doi: 10.1016/0009-8981(84)90195-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Hou Y. Y. Iron complexes and their reactivity in the bleomycin assay for radical-promoting loosely-bound iron. Free Radic Res Commun. 1986;2(3):143–151. doi: 10.3109/10715768609088066. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Shute D. J. Iron--dioxygen-dependent changes to the biological activities of bleomycin. J Inorg Biochem. 1981 Dec;15(4):349–357. doi: 10.1016/s0162-0134(00)80238-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Xiao Change F. Protection of iron-catalysed the radical damage to DNA and lipids by copper (II) bleomycin. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1354–1360. doi: 10.1016/0006-291x(81)90768-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Blake D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):659–671. doi: 10.1098/rstb.1985.0171. [DOI] [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Melnyk D. L., Horwitz S. B., Peisach J. Redox potential of iron-bleomycin. Biochemistry. 1981 Sep 1;20(18):5327–5331. doi: 10.1021/bi00521a036. [DOI] [PubMed] [Google Scholar]
- OSAKI S., MCDERMOTT J. A., FRIEDEN E. PROOF FOR THE ASCORBATE OXIDASE ACTIVITY OF CERULOPLASMIN. J Biol Chem. 1964 Oct;239:3570–3575. [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Rowley D., Gutteridge J. M., Blake D., Farr M., Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1984 Jun;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Scudder P. R., Al-Timimi D., McMurray W., White A. G., Zoob B. C., Dormandy T. L. Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis. 1978 Feb;37(1):67–70. doi: 10.1136/ard.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]


