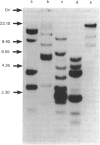
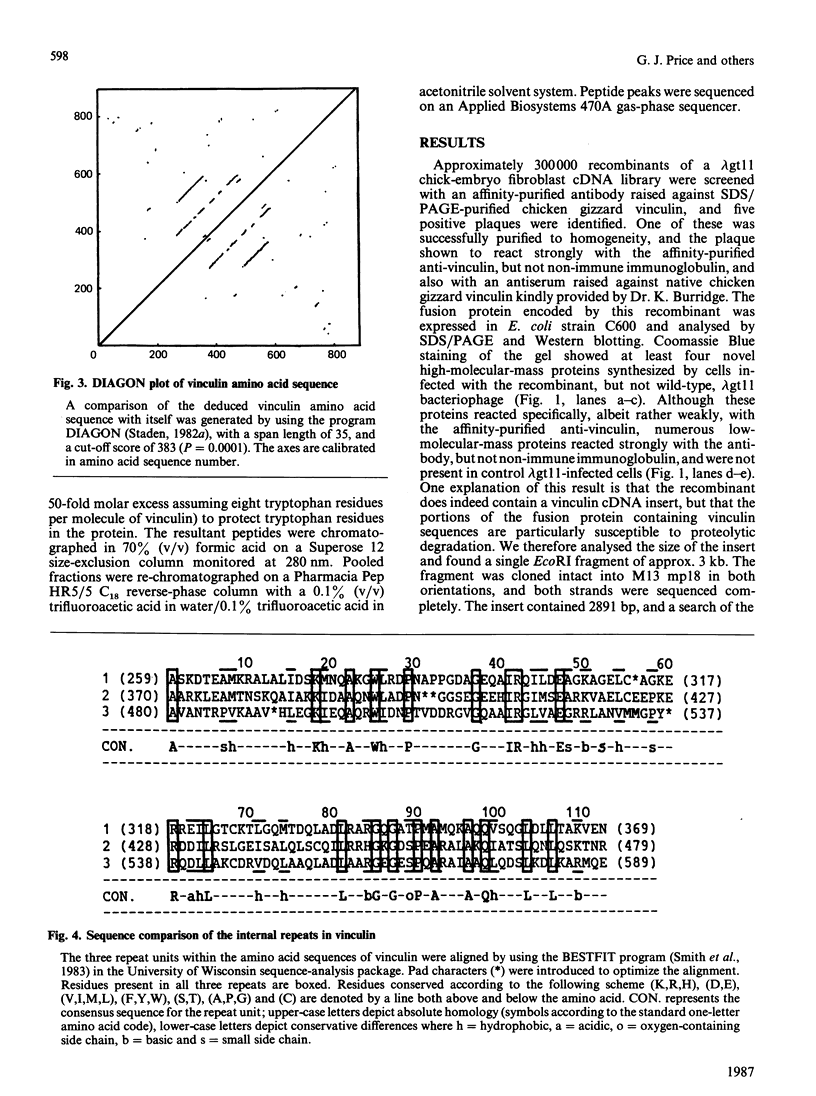
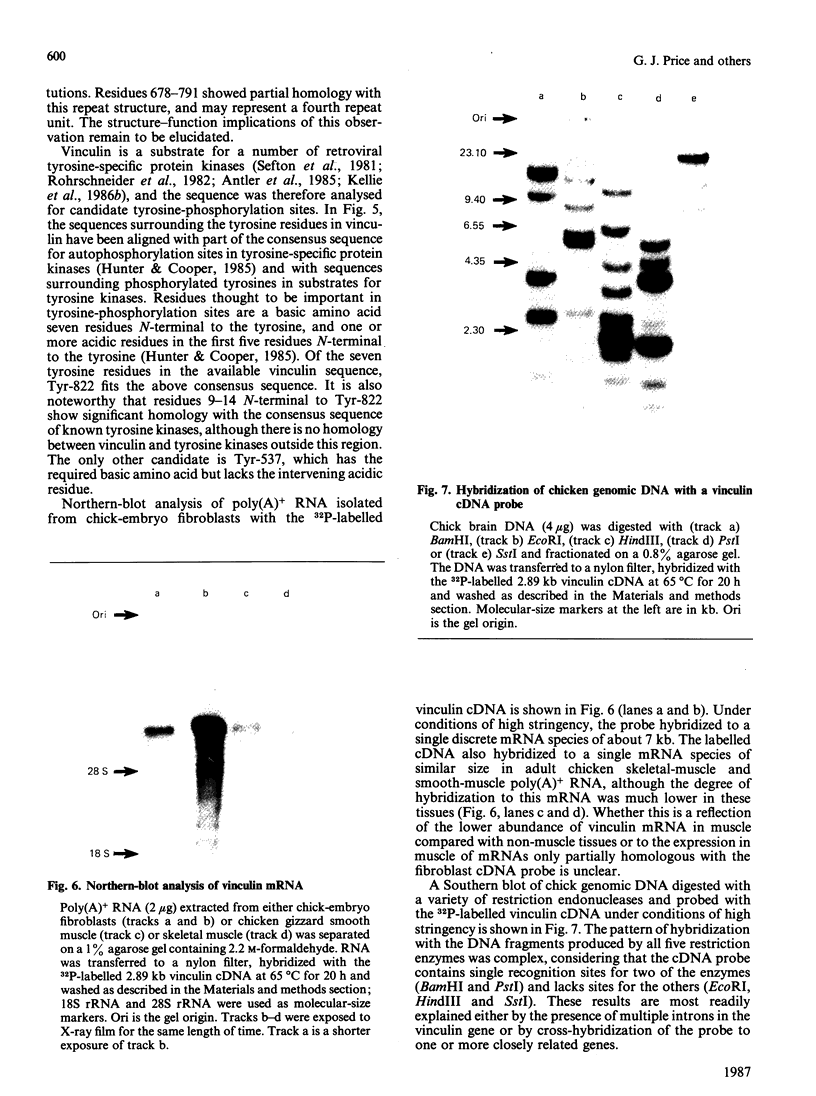
Abstract
A chick-embryo fibroblast lambda gt11 cDNA library was screened with affinity-purified antibodies to chick gizzard vinculin. One recombinant was purified to homogeneity and the fusion protein expressed in Escherichia coli strain C600. The fusion protein was unstable, but polypeptides that reacted with vinculin antibodies, but not non-immune immunoglobulin, were detected by Western blotting. The recombinant contained a single EcoRI fragment of 2891 bp with a single open reading frame. The deduced protein sequence could be aligned with that of six CNBr-cleavage peptides and two tryptic peptides derived from chicken gizzard vinculin. AUG-247 has tentatively been identified as the initiation codon, as it is contained within the consensus sequence for initiation sites of higher eukaryotes. The cDNA lacks 3' sequence and encodes 74% of the vinculin sequence, presuming the molecular mass of vinculin to be 130,000 Da. Analysis of the deduced sequence showed no homologies with other protein sequences, but it does display a triple internal repeat of 112 amino acid residues covering residues 259-589. The sequences surrounding the seven tyrosine residues in the available sequence were aligned with the tyrosine autophosphorylation consensus sequence found in protein tyrosine kinases. Tyr-822 showed a good match to this consensus, and may represent one of the two major sites of tyrosine phosphorylation by pp60v-sre. Northern blots showed that the 2.89 kb vinculin cDNA hybridized to one size of mRNA (approx. 7 kb) in chick-embryo fibroblasts, chick smooth muscle and chick skeletal muscle. Southern blots revealed multiple hybridizing bands in genomic DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antler A. M., Greenberg M. E., Edelman G. M., Hanafusa H. Increased phosphorylation of tyrosine in vinculin does not occur upon transformation by some avian sarcoma viruses. Mol Cell Biol. 1985 Jan;5(1):263–267. doi: 10.1128/mcb.5.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. D., Davison M. D., Jones P., Patel B., Critchley D. R. Isolation and characterization of a cDNA encoding a chick alpha-actinin. J Biol Chem. 1987 Feb 25;262(6):2558–2561. [PubMed] [Google Scholar]
- Bennett J. P., Zaner K. S., Stossel T. P. Isolation and some properties of macrophage alpha-actinin: evidence that it is not an actin gelling protein. Biochemistry. 1984 Oct 9;23(21):5081–5086. doi: 10.1021/bi00316a039. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Shaw W. V. The use of synthetic oligonucleotides with universal templates for rapid DNA sequencing: results with staphylococcal replicon pC221. EMBO J. 1985 Feb;4(2):561–568. doi: 10.1002/j.1460-2075.1985.tb03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn P., Burger M. M. The cytoskeletal protein vinculin contains transformation-sensitive, covalently bound lipid. Science. 1987 Jan 23;235(4787):476–479. doi: 10.1126/science.3099391. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983 Aug;97(2):359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. An interaction between vinculin and talin. Nature. 1984 Apr 19;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Chen Z. Q., Ulsh L. S., DuBois G., Shih T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J Virol. 1985 Nov;56(2):607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Dalgleish R., Williams G., Hawkins J. R. Length polymorphism in the pro alpha 2(I) collagen gene: an alternative explanation in a case of Marfan syndrome. Hum Genet. 1986 May;73(1):91–92. doi: 10.1007/BF00292673. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Franz H. Identification of actin-, alpha-actinin-, and vinculin-containing plaques at the lateral membrane of epithelial cells. J Cell Biol. 1986 May;102(5):1843–1852. doi: 10.1083/jcb.102.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol. 1986 May;102(5):1738–1747. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. R., Robson R. M., Stromer M. H. Properties of smooth muscle vinculin. J Biol Chem. 1984 Mar 25;259(6):3916–3924. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. XI. Peptide ordering procedures and the complete sequence. J Biol Chem. 1978 Aug 10;253(15):5521–5525. [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Geiger B. Microheterogeneity of avian and mammalian vinculin distinctive subcellular distribution of different isovinculins. J Mol Biol. 1982 Aug 25;159(4):685–701. doi: 10.1016/0022-2836(82)90108-5. [DOI] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Heath J. Cell biology. Finding the missing links. Nature. 1986 Apr 10;320(6062):484–485. doi: 10.1038/320484a0. [DOI] [PubMed] [Google Scholar]
- Herman B., Pledger W. J. Platelet-derived growth factor-induced alterations in vinculin and actin distribution in BALB/c-3T3 cells. J Cell Biol. 1985 Apr;100(4):1031–1040. doi: 10.1083/jcb.100.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T., Wagner D. D. Relationships between microfilaments, cell-substratum adhesion, and fibronectin. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):659–670. doi: 10.1101/sqb.1982.046.01.062. [DOI] [PubMed] [Google Scholar]
- Ito S., Werth D. K., Richert N. D., Pastan I. Vinculin phosphorylation by the src kinase. Interaction of vinculin with phospholipid vesicles. J Biol Chem. 1983 Dec 10;258(23):14626–14631. [PubMed] [Google Scholar]
- Kellie S., Patel B., Mitchell A., Critchley D. R., Wigglesworth N. M., Wyke J. A. Comparison of the relative importance of tyrosine-specific vinculin phosphorylation and the loss of surface-associated fibronectin in the morphology of cells transformed by Rous sarcoma virus. J Cell Sci. 1986 Jun;82:129–142. doi: 10.1242/jcs.82.1.129. [DOI] [PubMed] [Google Scholar]
- Kellie S., Patel B., Wigglesworth N. M., Critchley D. R., Wyke J. A. The use of Rous sarcoma virus transformation mutants with differing tyrosine kinase activities to study the relationships between vinculin phosphorylation, pp60v-src location and adhesion plaque integrity. Exp Cell Res. 1986 Jul;165(1):216–228. doi: 10.1016/0014-4827(86)90546-x. [DOI] [PubMed] [Google Scholar]
- Kellie S., Wigglesworth N. M. The cytoskeletal protein vinculin is acylated by myristic acid. FEBS Lett. 1987 Mar 23;213(2):428–432. doi: 10.1016/0014-5793(87)81536-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R., Houlker C., Reinach F. C., Talbot K. The mRNA and RNA-copy pseudogenes encoding TM30nm, a human cytoskeletal tropomyosin. Nucleic Acids Res. 1986 Nov 11;14(21):8413–8426. doi: 10.1093/nar/14.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Meigs J. B., Wang Y. L. Reorganization of alpha-actinin and vinculin induced by a phorbol ester in living cells. J Cell Biol. 1986 Apr;102(4):1430–1438. doi: 10.1083/jcb.102.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam L. M. Electron microscopy of rotary shadowed vinculin and vinculin complexes. J Mol Biol. 1985 Aug 5;184(3):543–545. doi: 10.1016/0022-2836(85)90301-8. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Niggli V., Dimitrov D. P., Brunner J., Burger M. M. Interaction of the cytoskeletal component vinculin with bilayer structures analyzed with a photoactivatable phospholipid. J Biol Chem. 1986 May 25;261(15):6912–6918. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pappin D. J., Findlay J. B. Sequence variability in the retinal-attachment domain of mammalian rhodopsins. Biochem J. 1984 Feb 1;217(3):605–613. doi: 10.1042/bj2170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L., Rosok M., Shriver K. Mechanism of transformation by Rous sarcoma virus: events within adhesion plaques. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):953–965. doi: 10.1101/sqb.1982.046.01.089. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Meta-vinculin--a vinculin-related protein with solubility properties of a membrane protein. Nature. 1982 Dec 9;300(5892):533–535. doi: 10.1038/300533a0. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S., Sadler J. R. Statistical characterization of nucleic acid sequence functional domains. Nucleic Acids Res. 1983 Apr 11;11(7):2205–2220. doi: 10.1093/nar/11.7.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth D. K., Pastan I. Vinculin phosphorylation in response to calcium and phorbol esters in intact cells. J Biol Chem. 1984 Apr 25;259(8):5264–5270. [PubMed] [Google Scholar]