Abstract
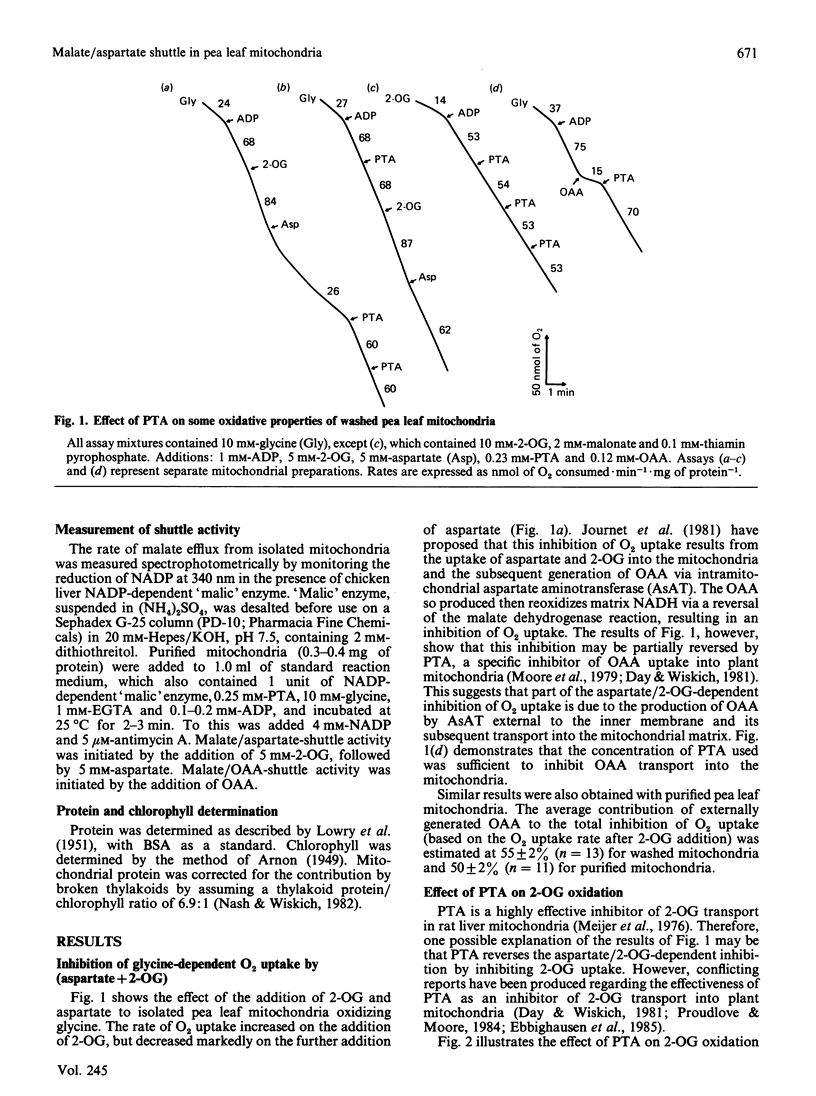
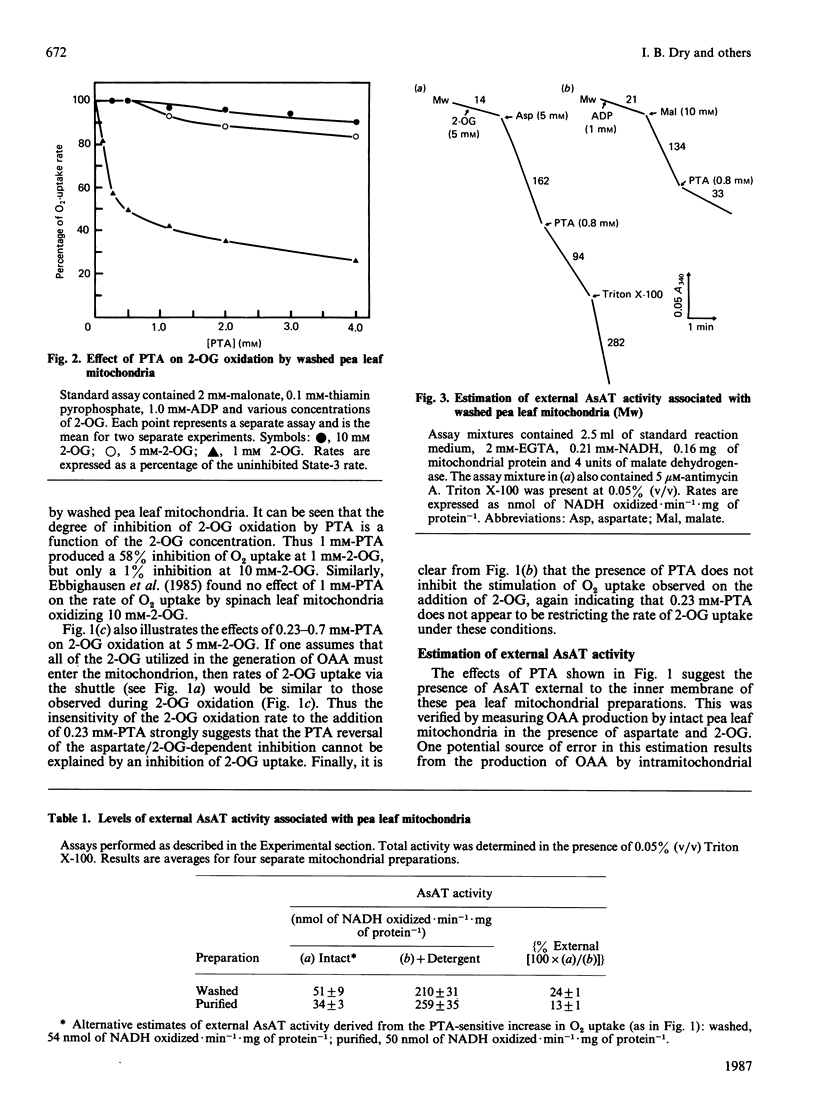
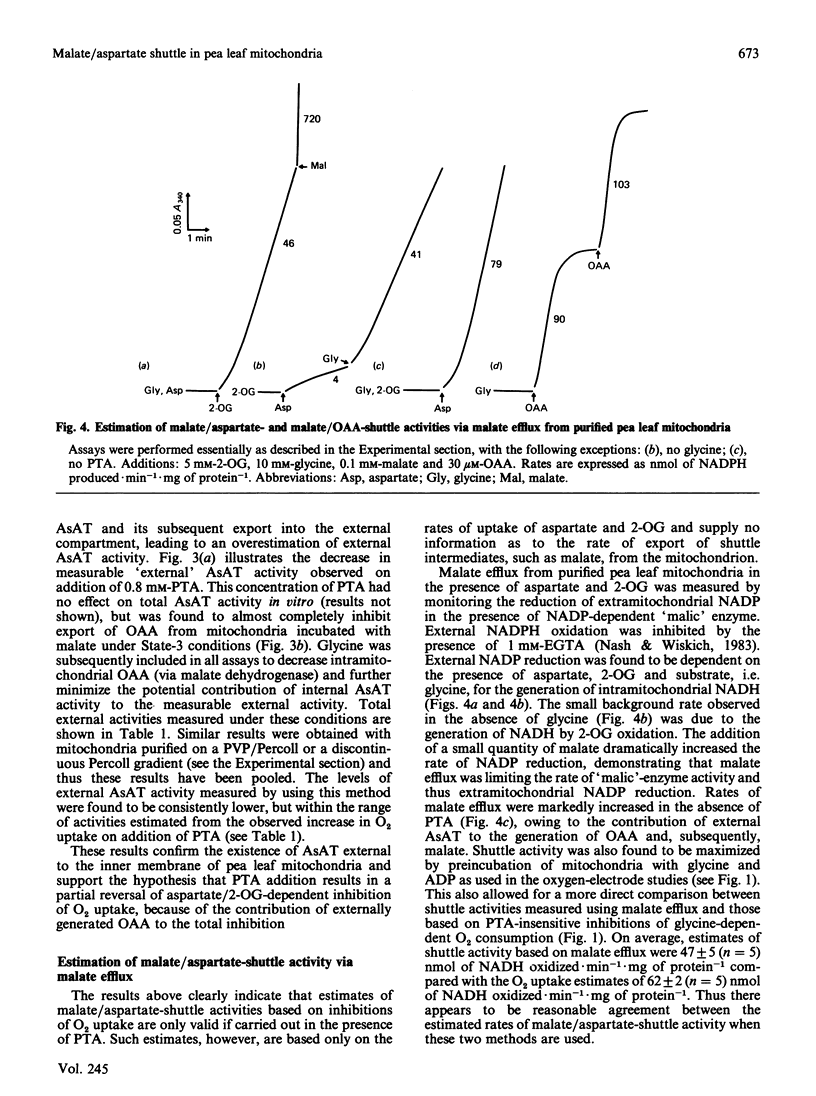
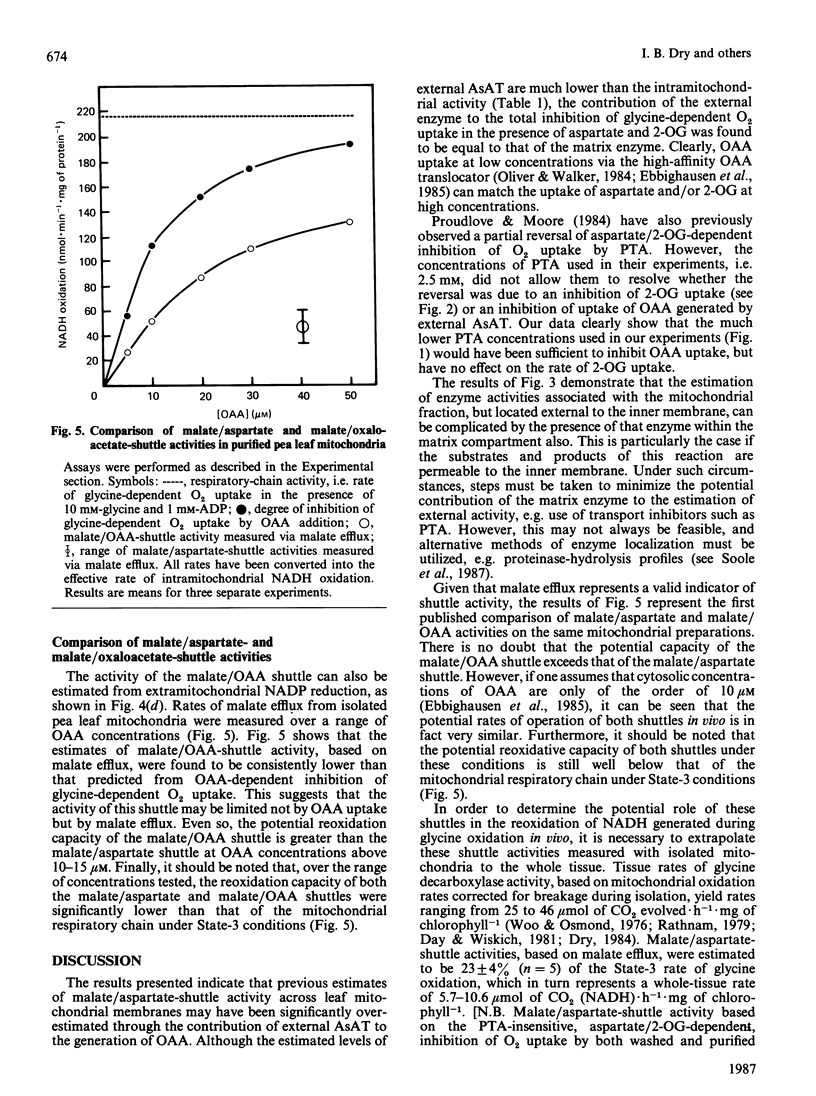
A method is presented for the preparation of pure phthalonic acid (PTA) in high yields. This PTA was used to determine the capacity of the malate/aspartate shuttle in pea (Pisum sativum) leaf mitochondria. The inhibition of glycine-dependent O2 uptake in the combined presence of 5 mM-aspartate and 5 mM-2-oxoglutarate (2-OG) was decreased by 55 +/- 22% (n = 13) in washed and 50 +/- 2% (n = 11) in purified mitochondria by 0.23 mM-PTA. This concentration of PTA had no effect on the oxidation of 5 mM-2-OG, suggesting that part of the observed inhibition of O2 uptake in the presence of aspartate and 2-OG was due to the production of oxaloacetate (OAA) by aspartate aminotransferase external to the mitochondrial inner membrane. Levels of external aspartate aminotransferase were estimated to be 24 +/- 1% (n = 4) and 13 +/- 1% (n = 4) of the total mitochondrial activity in washed and purified mitochondria respectively. Malate/aspartate-shuttle activity was estimated directly by measuring rates of malate efflux from isolated mitochondria and was found to match estimates of shuttle activity based on the PTA-insensitive inhibition of O2 uptake. Comparisons of malate/aspartate- and malate/OAA-shuttle activities indicated potentially similar rates of NADH export from pea leaf mitochondria under conditions in vivo. These extrapolated to whole-tissue rates of 5-11 mumol of NADH.h-1.mg of chlorophyll-1. The potential role of the malate/aspartate shuttle in the support of photorespiratory glycine oxidation in leaf tissue is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier D., Douce R. Interactions between Mitochondria and Chloroplasts in Cells: I. Action of Cyanide and of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea on the Spore of Funaria hygrometrica. Plant Physiol. 1976 Mar;57(3):400–402. doi: 10.1104/pp.57.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A. Malate Decarboxylation by Kalanchoë daigremontiana Mitochondria and Its Role in Crassulacean Acid Metabolism. Plant Physiol. 1980 Apr;65(4):675–679. doi: 10.1104/pp.65.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Neuburger M., Douce R. Role of Glutamate-oxaloacetate Transaminase and Malate Dehydrogenase in the Regeneration of NAD for Glycine Oxidation by Spinach leaf Mitochondria. Plant Physiol. 1981 Mar;67(3):467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meijer A. J., von Woerkom G. M., Eggelte T. A. Phthalonic acid, an inhibitor of alpha-oxoglutarate transport in mitochondria. Biochim Biophys Acta. 1976 Apr 9;430(1):53–61. doi: 10.1016/0005-2728(76)90221-8. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Nash D., Wiskich J. T. Properties of substantially chlorophyll-free pea leaf mitochondria prepared by sucrose density gradient separation. Plant Physiol. 1983 Mar;71(3):627–634. doi: 10.1104/pp.71.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J., Walker G. H. Characterization of the transport of oxaloacetate by pea leaf mitochondria. Plant Physiol. 1984 Oct;76(2):409–413. doi: 10.1104/pp.76.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Provisions of reductant for the hydroxypyruvate to glycerate conversion in leaf peroxisomes : a critical evaluation of the proposed malate/aspartate shuttle. Plant Physiol. 1983 Jul;72(3):728–734. doi: 10.1104/pp.72.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


