Abstract
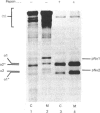
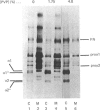
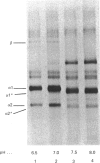
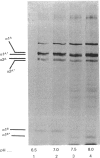
In human skin fibroblast cultures a fraction of the procollagen that was processed to collagen and remained in the cell layer was further proteolytically modified by removal of both N- and C-terminal telopeptides. The proteolytic activity was associated with the cell layer, since secreted collagens were found always to contain intact telopeptides. The inclusion of neutral polymers, which caused the accumulation of the collagen in the cell layer [Bateman, Cole, Pillow & Ramshaw (1986) J. Biol. Chem. 261, 4198-4203], made the telopeptide cleavage more apparent in those cells which expressed the proteolytic activity. The extent of this cleavage was variable from cell culture to cell culture and between experiments with the same fibroblast line. The proteolytic activity was pH-dependent; cleavage was greatest at a culture-medium pH of 7.5 and 8.0 and was completely inhibited at a culture-medium pH of 7.0 and 6.5. The activity was significantly inhibited by soybean trypsin inhibitor, an elastase-specific inhibitor (N-acetylalanylalanylprolylvalylchloromethane) and the thrombin inhibitor hirudin. This cell-associated proteolytic activity may play a role in collagen degradation by removing the telopeptides, which are the primary sites of collagen cross-linking, thus destabilizing the collagen matrix sufficiently to render it susceptible to further proteolytic breakdown.
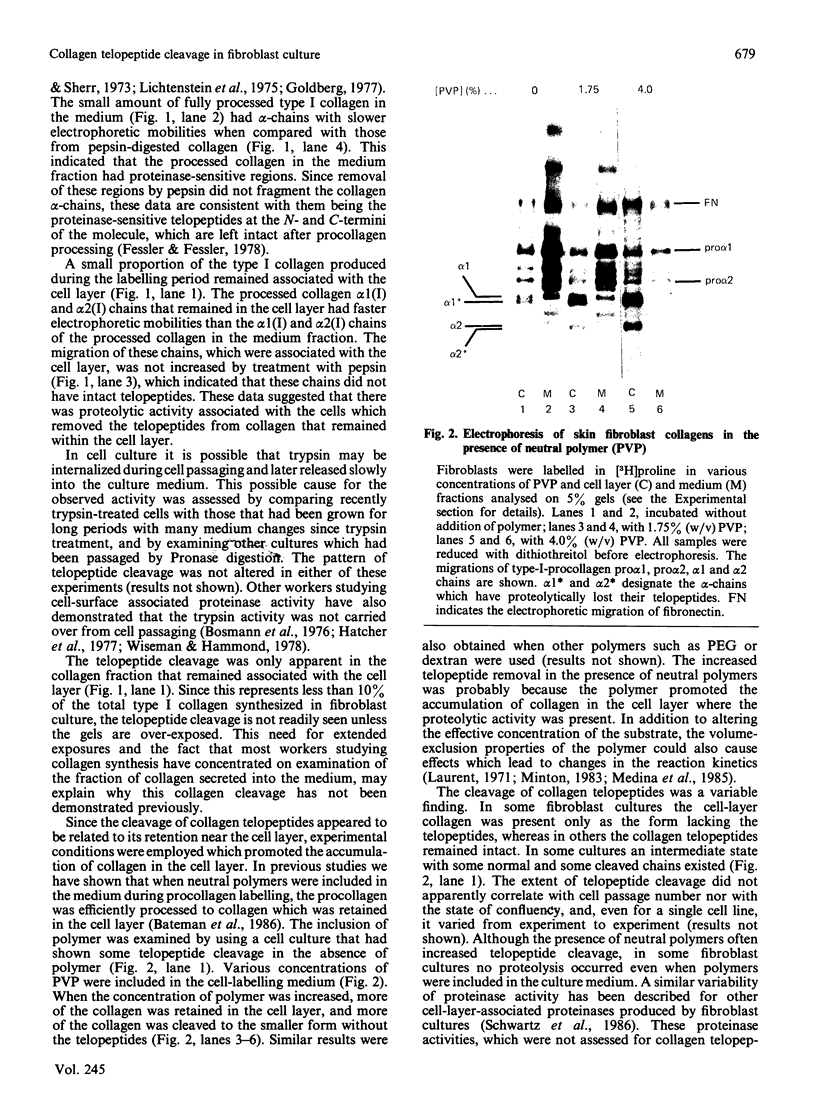
Full text
PDF

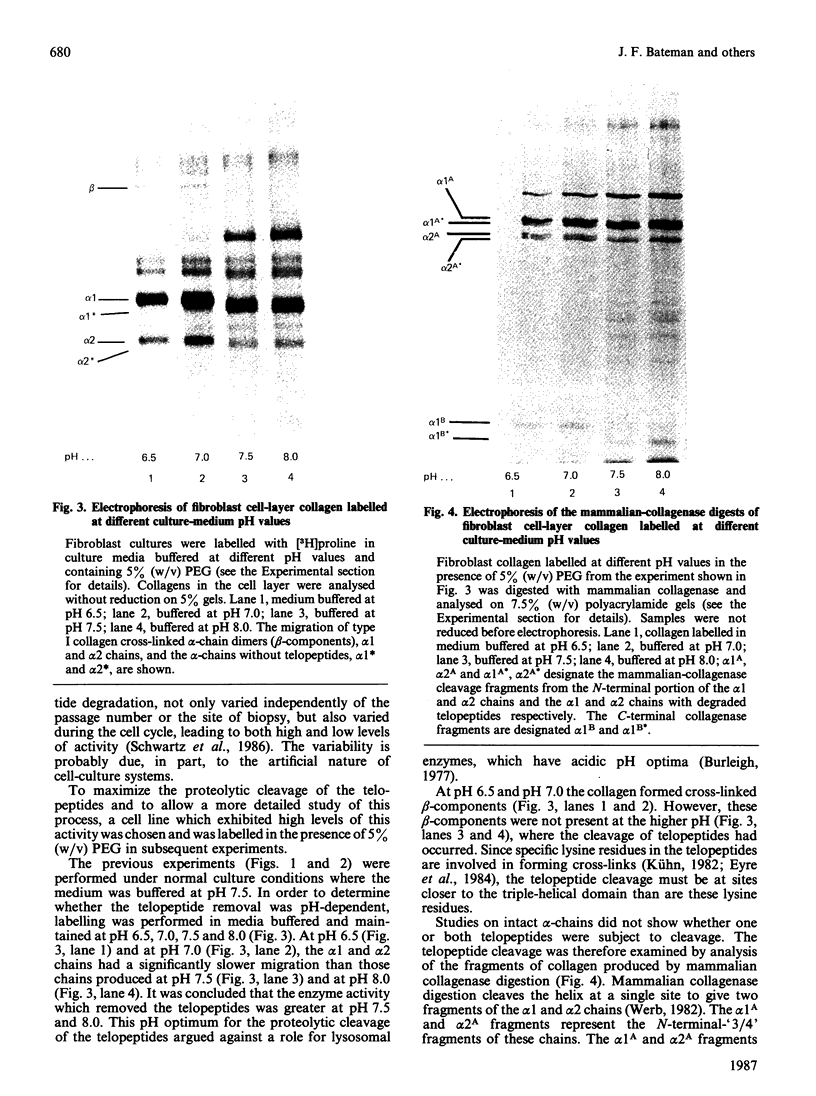

Images in this article
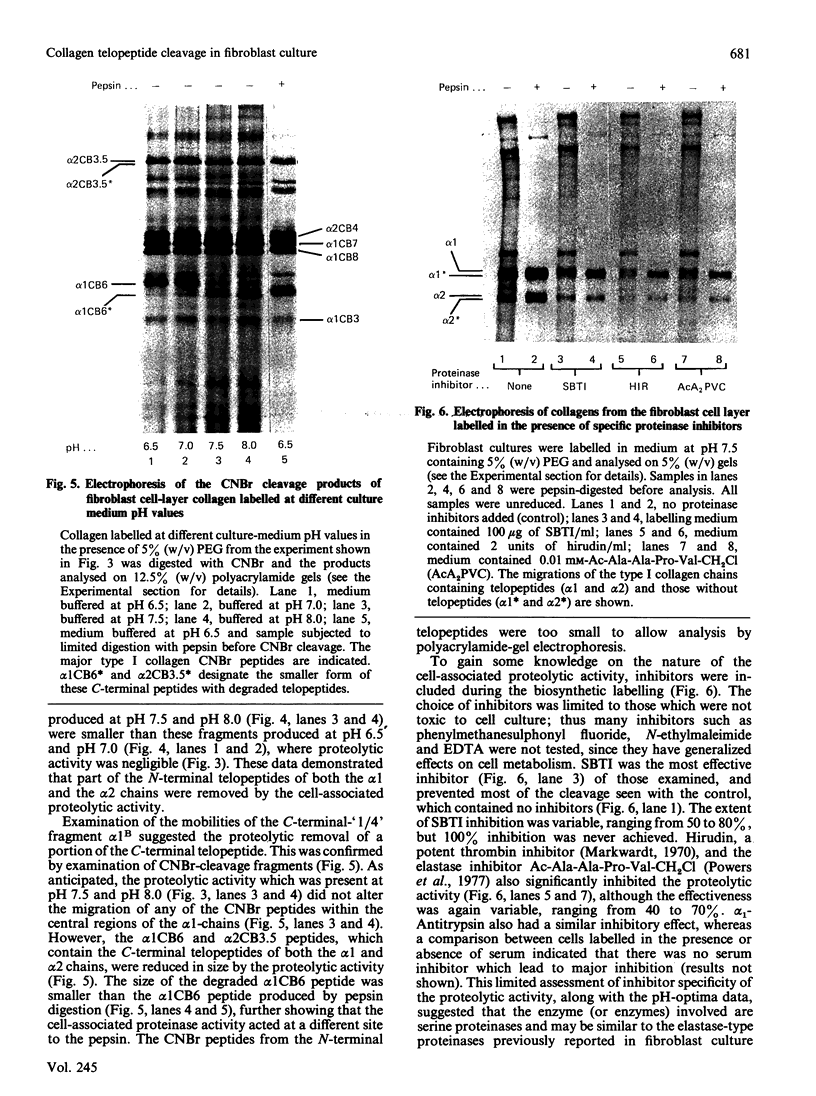
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman J. F., Cole W. G., Pillow J. J., Ramshaw J. A. Induction of procollagen processing in fibroblast cultures by neutral polymers. J Biol Chem. 1986 Mar 25;261(9):4198–4203. [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Peterkofsky B. Mechanisms of Kirsten murine sarcoma virus transformation-induced changes in the collagen phenotype and synthetic rate of BALB 3T3 cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6028–6032. doi: 10.1073/pnas.78.10.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Guthell R. L., Jr, Case K. R. Loss of a critical neutral protease in ageing WI-38 cells. Nature. 1976 Jun 10;261(5560):499–501. doi: 10.1038/261499a0. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Paz M. A., Gallop P. M. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Godeau G., Frances C., Hornebeck W., Brechemier D., Robert L. Isolation and partial characterization of an elastase-type protease in human vulva fibroblasts: its possible involvement in vulvar elastic tissue destruction of patients with lichen sclerosus et atrophicus. J Invest Dermatol. 1982 Apr;78(4):270–275. doi: 10.1111/1523-1747.ep12506899. [DOI] [PubMed] [Google Scholar]
- Goldberg B. Kinetics of processing of type I and type III procollagens in fibroblast cultures. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3322–3325. doi: 10.1073/pnas.74.8.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Sherr C. J. Secretion and extracellular processing of procollagen by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1973 Feb;70(2):361–365. doi: 10.1073/pnas.70.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper L., Scott G. K., Seow H. F. Antibody affinity chromatography of human proteinases and related proteins. Comp Biochem Physiol B. 1984;78(1):231–235. doi: 10.1016/0305-0491(84)90175-5. [DOI] [PubMed] [Google Scholar]
- Hatcher V. B., Oberman M. S., Wertheim M. S., Rhee C. Y., Tsien G., Burk P. G. The relationship between surface protease activity and the rate of cell proliferation in normal and transformed cells. Biochem Biophys Res Commun. 1976 May 23;76(2):602–608. doi: 10.1016/0006-291x(77)90766-5. [DOI] [PubMed] [Google Scholar]
- Ku G. S., Quigley J. P., Sultzer B. M. The inhibition of the mitogenic stimulation of B lymphocytes by a serine protease inhibitor: commitment to proliferation correlates with an enhanced expression of a cell-associated arginine-specific serine enzyme. J Immunol. 1983 Nov;131(5):2494–2499. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. Enzyme reactions in polymer media. Eur J Biochem. 1971 Aug 25;21(4):498–506. doi: 10.1111/j.1432-1033.1971.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Failure of human rheumatoid synovial collagenase to degrade either normal or rheumatoid arthritic polymeric collagen. Biochim Biophys Acta. 1971 Oct;251(1):109–118. doi: 10.1016/0005-2795(71)90067-5. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Medina R., Aragón J. J., Sols A. Effect of polyethylene glycol on the kinetic behaviour of pyruvate kinase and other potentially regulatory liver enzymes. FEBS Lett. 1985 Jan 21;180(1):77–80. doi: 10.1016/0014-5793(85)80235-0. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Scott G. K. Growth inhibition of normal, tumour, and transformed cells by antibodies to a cell-surface proteinase. Biosci Rep. 1983 Jan;3(1):47–51. doi: 10.1007/BF01121570. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Tuderman L. Posttranslational enzymes in the biosynthesis of collagen: extracellular enzymes. Methods Enzymol. 1982;82(Pt A):305–319. doi: 10.1016/0076-6879(82)82068-5. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Paller A. S., Lizak P. P., Pearson R. W. Elastase and neutral cathepsin production by human fibroblasts: effect of culture conditions on synthesis and secretion. J Invest Dermatol. 1986 Jan;86(1):63–68. doi: 10.1111/1523-1747.ep12283833. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Goldberg H. A., Dodd C. M. A neutral proteinase from human gingival fibroblasts active against the c-terminal cross-linking region of type I collagen. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1064–1070. doi: 10.1016/0006-291x(83)90670-8. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Griffin M. M., Itzhaki S. Inhibition of free and bound trypsin-like enzymes. Eur J Biochem. 1982 Aug;126(2):311–318. doi: 10.1111/j.1432-1033.1982.tb06780.x. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Harris E. D., Jr, Siegel R. C. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979 Sep 1;181(3):639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Javed T., Miller R. L. Human gingival fibroblast collagenase: purification and properties of precursor and active forms. Coll Relat Res. 1984 Mar;4(2):129–152. doi: 10.1016/s0174-173x(84)80021-7. [DOI] [PubMed] [Google Scholar]