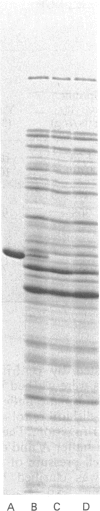
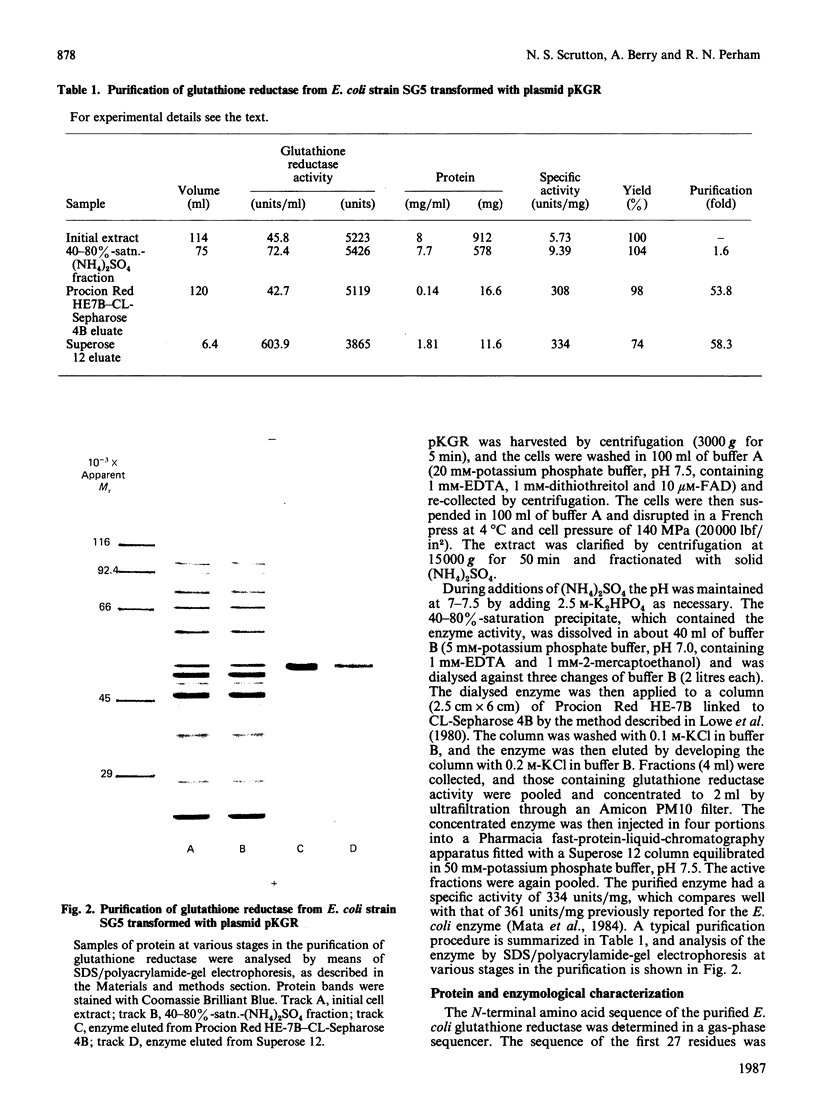
Abstract
An expression vector, pKGR, for the gor gene from Escherichia coli encoding glutathione reductase was constructed by subcloning of an AvaII fragment of the Clarke & Carbon bank plasmid pGR [Greer & Perham (1986) Biochemistry 25, 2736-2742] into the plasmid pKK223-3. The expression of glutathione reductase from the plasmid pKGR was found to have been successfully placed under the control of the tac promoter. Transformation of E. coli cells with this plasmid resulted in 100-200-fold increase in glutathione reductase activity in cell-free extracts. A rapid purification procedure for the enzyme, based on affinity chromatography on Procion Red HE-7B-CL-Sepharose 4B, was developed. The purified enzyme was homogeneous as judged by SDS/polyacrylamide-gel electrophoresis, and all its properties were consistent with the DNA sequence of the gene [Greer & Perham (1986) Biochemistry 25, 2736-2742] and with those previously reported for E. coli glutathione reductase [Mata, Pinto & Lopez-Barea (1984) Z. Naturforsch. C. Biosci. 39, 908-915]. These experiments have enabled an investigation of the protein chemical and mechanistic properties of the enzyme by site-directed mutagenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allington W. B., Cordry A. L., McCullough G. A., Mitchell D. E., Nelson J. W. Electrophoretic concentration of macromolecules. Anal Biochem. 1978 Mar;85(1):188–196. doi: 10.1016/0003-2697(78)90289-0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Ford S. J., Pridmore R. D., Fritzinger D. C. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983 Aug 16;22(17):4089–4095. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- Flinta C., Persson B., Jörnvall H., von Heijne G. Sequence determinants of cytosolic N-terminal protein processing. Eur J Biochem. 1986 Jan 2;154(1):193–196. doi: 10.1111/j.1432-1033.1986.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Walsh C. T. Mercuric reductase: homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry. 1983 Aug 16;22(17):4082–4088. doi: 10.1021/bi00286a014. [DOI] [PubMed] [Google Scholar]
- Fox B., Walsh C. T. Mercuric reductase. Purification and characterization of a transposon-encoded flavoprotein containing an oxidation-reduction-active disulfide. J Biol Chem. 1982 Mar 10;257(5):2498–2503. [PubMed] [Google Scholar]
- Greer S., Perham R. N. Glutathione reductase from Escherichia coli: cloning and sequence analysis of the gene and relationship to other flavoprotein disulfide oxidoreductases. Biochemistry. 1986 May 6;25(9):2736–2742. doi: 10.1021/bi00357a069. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Pyridine nucleotide - disulfide oxidoreductases. Experientia Suppl. 1980;36:149–180. doi: 10.1007/978-3-0348-5419-1_5. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R. L., Blatterspiel R., Saleh M., Schiltz E., Schirmer R. H., Untucht-Grau R. Glutathione reductase from human erythrocytes. The sequences of the NADPH domain and of the interface domain. Eur J Biochem. 1982 Jan;121(2):259–267. doi: 10.1111/j.1432-1033.1982.tb05780.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Hans M., Spibey N., Drabble W. T. The purification of inosine 5'-monophosphate dehydrogenase from Escherichia coli by affinity chromatography on immobilized Procion dyes. Anal Biochem. 1980 May 1;104(1):23–28. doi: 10.1016/0003-2697(80)90271-7. [DOI] [PubMed] [Google Scholar]
- Mata A. M., Pinto M. C., López-Barea J. Purification by affinity chromatography of glutathione reductase (EC 1.6.4.2) from Escherichia coli and characterization of such enzyme. Z Naturforsch C. 1984 Sep-Oct;39(9-10):908–915. doi: 10.1515/znc-1984-9-1009. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Packman L. C., Perham R. N. An amino acid sequence in the active site of lipoamide dehydrogenase from Bacillus stearothermophilus. FEBS Lett. 1982 Mar 22;139(2):155–158. doi: 10.1016/0014-5793(82)80839-9. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Schulz G. E. The catalytic mechanism of glutathione reductase as derived from x-ray diffraction analyses of reaction intermediates. J Biol Chem. 1983 Feb 10;258(3):1752–1757. [PubMed] [Google Scholar]
- Perham R. N., Harrison R. A., Brown J. P. The lipoamide dehydrogenase component of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem Soc Trans. 1978;6(1):47–50. doi: 10.1042/bst0060047. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schultz P. G., Au K. G., Walsh C. T. Directed mutagenesis of the redox-active disulfide in the flavoenzyme mercuric ion reductase. Biochemistry. 1985 Nov 19;24(24):6840–6848. doi: 10.1021/bi00345a016. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Lewis H. M., Darlison M. G., Guest J. R. Nucleotide sequence of the lipoamide dehydrogenase gene of Escherichia coli K12. Eur J Biochem. 1983 Oct 3;135(3):519–527. doi: 10.1111/j.1432-1033.1983.tb07683.x. [DOI] [PubMed] [Google Scholar]
- Thieme R., Pai E. F., Schirmer R. H., Schulz G. E. Three-dimensional structure of glutathione reductase at 2 A resolution. J Mol Biol. 1981 Nov 15;152(4):763–782. doi: 10.1016/0022-2836(81)90126-1. [DOI] [PubMed] [Google Scholar]
- Waye M. M., Winter G., Wilkinson A. J., Fersht A. R. Deletion mutagenesis using an 'M13 splint': the N-terminal structural domain of tyrosyl-tRNA synthetase (B. stearothermophilus) catalyses the formation of tyrosyl adenylate. EMBO J. 1983;2(10):1827–1829. doi: 10.1002/j.1460-2075.1983.tb01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. H., Jr, Arscott L. D., Schulz G. E. Amino acid sequence homology between pig heart lipoamide dehydrogenase and human erythrocyte glutathione reductase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2199–2201. doi: 10.1073/pnas.79.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]