Abstract
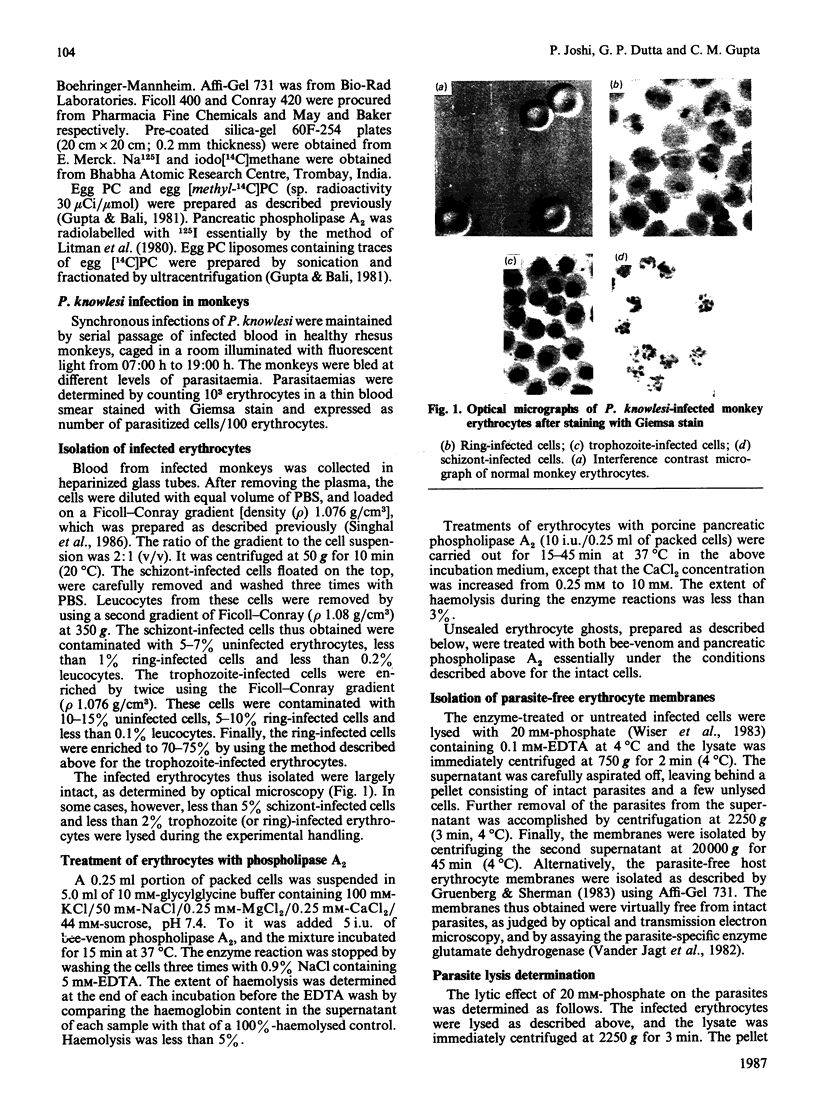
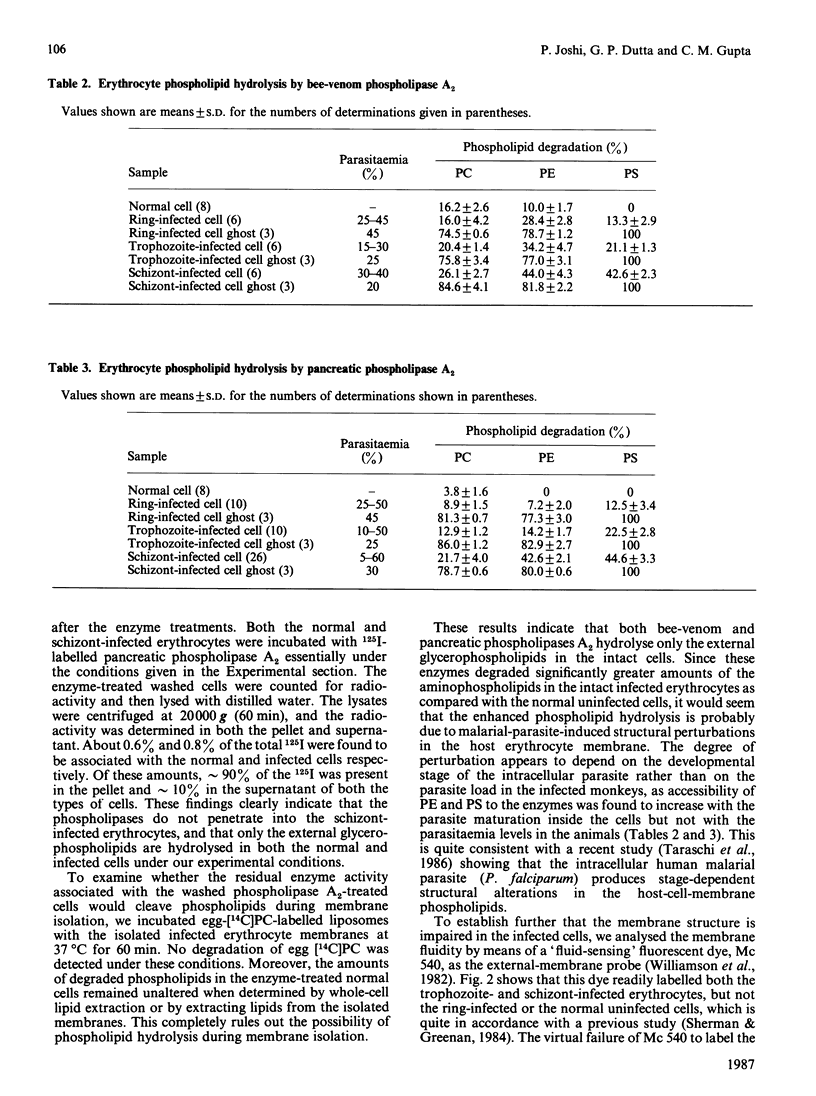
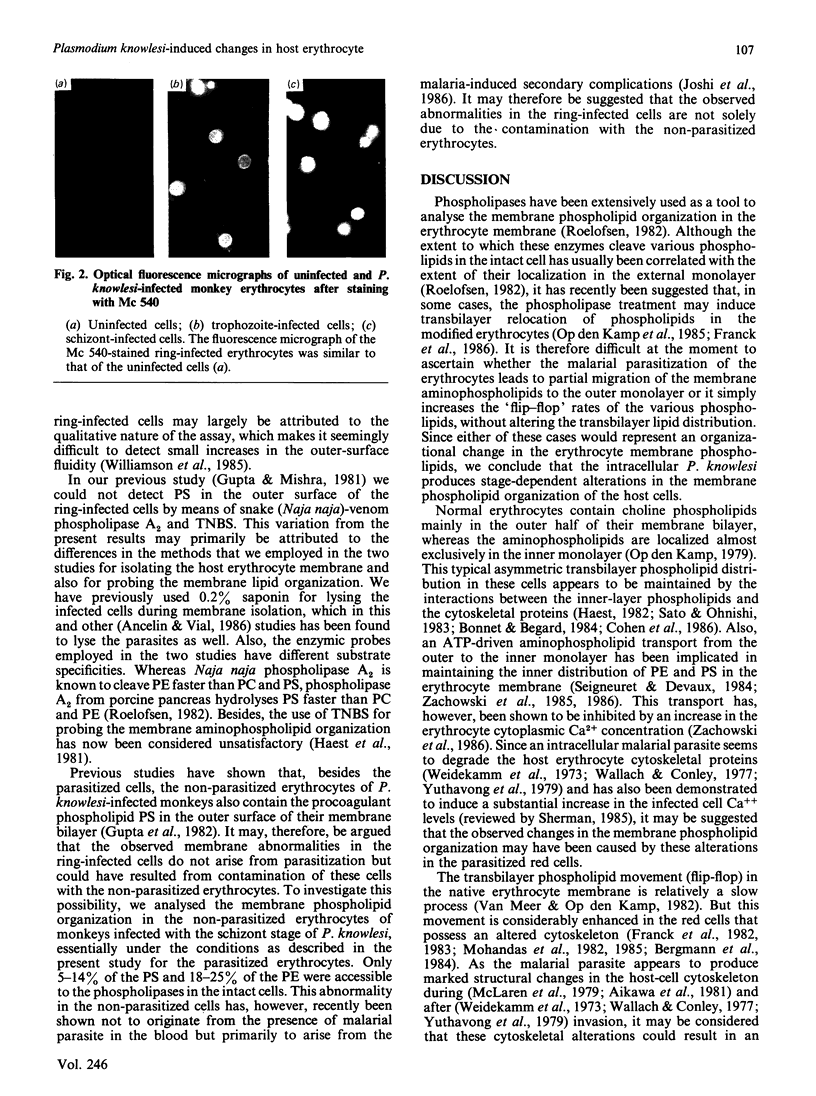
The membrane phospholipid organization in monkey erythrocytes harbouring different developmental stages of the simian malarial parasite Plasmodium knowlesi was studied using phospholipase A2 from two different sources and Merocyanine 540 as the external-membrane probes. Experiments were done to confirm that the phospholipases did not penetrate into the infected cells or hydrolyse phospholipids during membrane isolation. The parasite-free erythrocyte membrane was isolated by differential centrifugation or by using the cationic beads Affi-Gel 731. The purity of the membranes was established by optical and electron microscopy, and by assaying the parasite-specific enzyme glutamate dehydrogenase. About 10% of the phosphatidylethanolamine and none of phosphatidylserine were hydrolysed by the phospholipases in intact normal monkey erythrocytes. However, accessibility of these aminophospholipids to the enzymes was significantly enhanced in the infected cells under identical conditions. The degree of this enhancement depended on the developmental stage of the intracellular parasite, but not on the parasitaemia levels in the infected monkeys, and increased with the parasite growth inside the cells. Analogously, Merocyanine 540 was found to label the trophozoite- or schizont-infected erythrocytes, but not the ring-infected or normal cells. These results demonstrate that the intracellular malarial parasite produces stage-dependent alterations in the membrane phospholipid organization of its host erythrocyte.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Aikawa M., Miller L. H., Rabbege J. R., Epstein N. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J Cell Biol. 1981 Oct;91(1):55–62. doi: 10.1083/jcb.91.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred D. R., Sterling C. R., Morse P. D., 2nd Increased fluidity of Plasmodium berghei-infected mouse red blood cell membranes detected by electron spin resonance spectroscopy. Mol Biochem Parasitol. 1983 Jan;7(1):27–39. doi: 10.1016/0166-6851(83)90114-7. [DOI] [PubMed] [Google Scholar]
- Ancelin M. L., Vial H. J. Choline kinase activity in Plasmodium-infected erythrocytes: characterization and utilization as a parasite-specific marker in malarial fractionation studies. Biochim Biophys Acta. 1986 Jan 3;875(1):52–58. doi: 10.1016/0005-2760(86)90010-x. [DOI] [PubMed] [Google Scholar]
- Bergmann W. L., Dressler V., Haest C. W., Deuticke B. Reorientation rates and asymmetry of distribution of lysophospholipids between the inner and outer leaflet of the erythrocyte membrane. Biochim Biophys Acta. 1984 May 30;772(3):328–336. doi: 10.1016/0005-2736(84)90150-0. [DOI] [PubMed] [Google Scholar]
- Bonnet D., Begard E. Interaction of anilinonaphtyl labeled spectrin with fatty acids and phospholipids: a fluorescence study. Biochem Biophys Res Commun. 1984 Apr 30;120(2):344–350. doi: 10.1016/0006-291x(84)91260-9. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Liu S. C., Derick L. H., Palek J. Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood. 1986 Oct;68(4):920–926. [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Franck P. F., Chiu D. T., Op den Kamp J. A., Lubin B., van Deenen L. L., Roelofsen B. Accelerated transbilayer movement of phosphatidylcholine in sickled erythrocytes. A reversible process. J Biol Chem. 1983 Jul 10;258(13):8436–8442. [PubMed] [Google Scholar]
- Franck P. F., Op den Kamp J. A., Roelofsen B., van Deenen L. L. Does diamide treatment of intact human erythrocytes cause a loss of phospholipid asymmetry? Biochim Biophys Acta. 1986 May 9;857(1):127–130. doi: 10.1016/0005-2736(86)90106-9. [DOI] [PubMed] [Google Scholar]
- Franck P. F., Roelofsen B., Op den Kamp J. A. Complete exchange of phosphatidylcholine from intact erythrocytes after protein crosslinking. Biochim Biophys Acta. 1982 Apr 23;687(1):105–108. doi: 10.1016/0005-2736(82)90176-6. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Kutner S., Krugliak M., Cabantchik Z. I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985 Mar;14(3):313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Sherman I. W. Isolation and characterization of the plasma membrane of human erythrocytes infected with the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1087–1091. doi: 10.1073/pnas.80.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C. M., Alam A., Mathur P. N., Dutta G. P. A new look at nonparasitized red cells of malaria-infected monkeys. Nature. 1982 Sep 16;299(5880):259–261. doi: 10.1038/299259a0. [DOI] [PubMed] [Google Scholar]
- Gupta C. M., Bali A. Carbamyl analogs of phosphatidylcholines. Synthesis, interaction with phospholipases and permeability behavior of their liposomes. Biochim Biophys Acta. 1981 Feb 23;663(2):506–515. doi: 10.1016/0005-2760(81)90178-8. [DOI] [PubMed] [Google Scholar]
- Gupta C. M., Mishra G. C. Transbilayer phospholipid asymmetry in Plasmodium knowlesi-infected host cell membrane. Science. 1981 May 29;212(4498):1047–1049. doi: 10.1126/science.7233198. [DOI] [PubMed] [Google Scholar]
- Haest C. W. Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta. 1982 Dec;694(4):331–352. doi: 10.1016/0304-4157(82)90001-6. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Deuticke B. Penetration of 2,4,5-trinitrobenzenesulfonate into human erythrocytes. Consequences for studies on phospholipid asymmetry. Biochim Biophys Acta. 1981 Jan 22;640(2):535–543. doi: 10.1016/0005-2736(81)90477-6. [DOI] [PubMed] [Google Scholar]
- Holz G. G., Jr Lipids and the malarial parasite. Bull World Health Organ. 1977;55(2-3):237–248. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Sawyer W. H. Changes in the membrane microviscosity of mouse red blood cells infected with Plasmodium berghei detected using n-(9-anthroyloxy) fatty acid fluorescent probes. Parasitology. 1980 Apr;80(2):331–342. doi: 10.1017/s0031182000000792. [DOI] [PubMed] [Google Scholar]
- Joshi P., Alam A., Chandra R., Puri S. K., Gupta C. M. Possible basis for membrane changes in nonparasitized erythrocytes of malaria-infected animals. Biochim Biophys Acta. 1986 Nov 6;862(1):220–222. doi: 10.1016/0005-2736(86)90486-4. [DOI] [PubMed] [Google Scholar]
- Kumar A., Gupta C. M. Red cell membrane abnormalities in chronic myeloid leukaemia. Nature. 1983 Jun 16;303(5918):632–633. doi: 10.1038/303632a0. [DOI] [PubMed] [Google Scholar]
- Kutner S., Ginsburg H., Cabantchik Z. I. Permselectivity changes in malaria (Plasmodium falciparum) infected human red blood cell membranes. J Cell Physiol. 1983 Feb;114(2):245–251. doi: 10.1002/jcp.1041140215. [DOI] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Bannister L. H., Trigg P. I., Butcher G. A. Freeze fracture studies on the interaction between the malaria parasite and the host erythrocyte in Plasmodium knowlesi infections. Parasitology. 1979 Aug;79(1):125–139. doi: 10.1017/s0031182000052021. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Rossi M., Bernstein S., Ballas S., Ravindranath Y., Wyatt J., Mentzer W. The structural organization of skeletal proteins influences lipid translocation across erythrocyte membrane. J Biol Chem. 1985 Nov 15;260(26):14264–14268. [PubMed] [Google Scholar]
- Mohandas N., Wyatt J., Mel S. F., Rossi M. E., Shohet S. B. Lipid translocation across the human erythrocyte membrane. Regulatory factors. J Biol Chem. 1982 Jun 10;257(11):6537–6543. [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Pollet S., Ermidou S., Le Saux F., Monge M., Baumann N. Microanalysis of brain lipids: multiple two-dimensional thin-layer chromatography. J Lipid Res. 1978 Sep;19(7):916–921. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Sato S. B., Ohnishi S. Interaction of a peripheral protein of the erythrocyte membrane, band 4.1, with phosphatidylserine-containing liposomes and erythrocyte inside-out vesicles. Eur J Biochem. 1983 Jan 17;130(1):19–25. doi: 10.1111/j.1432-1033.1983.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Phelps B. M., Waggoner A., Terada L., Williamson P. Binding of merocyanine 540 to normal and leukemic erythroid cells. Cell. 1980 Jun;20(2):321–328. doi: 10.1016/0092-8674(80)90618-2. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare P. G., Trigg P. I., Tappenden L. Some properties of membranes in the simian malaria parasite Plasmodium knowlesi. Ann Trop Med Parasitol. 1979 Aug;73(4):333–343. doi: 10.1080/00034983.1979.11687267. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Greenan J. R. Altered red cell membrane fluidity during schizogonic development of malarial parasites (Plasmodium falciparum and P. lophurae). Trans R Soc Trop Med Hyg. 1984;78(5):641–644. doi: 10.1016/0035-9203(84)90227-x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Membrane structure and function of malaria parasites and the infected erythrocyte. Parasitology. 1985 Dec;91(Pt 3):609–645. doi: 10.1017/s0031182000062843. [DOI] [PubMed] [Google Scholar]
- Singhal A., Bali A., Gupta C. M. Antibody-mediated targeting of liposomes to erythrocytes in whole blood. Biochim Biophys Acta. 1986 Jan 15;880(1):72–77. doi: 10.1016/0304-4165(86)90121-2. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., Parashar A., Hooks M., Rubin H. Perturbation of red cell membrane structure during intracellular maturation of Plasmodium falciparum. Science. 1986 Apr 4;232(4746):102–104. doi: 10.1126/science.3006251. [DOI] [PubMed] [Google Scholar]
- Trigg P. I., Hirst S. I., Shakespeare P. G., Tappenden L. Labelling of membrane glycoprotein in erythrocytes infected with Plasmodium knowlesi. Bull World Health Organ. 1977;55(2-3):205–209. [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt D. L., Intress C., Heidrich J. E., Mrema J. E., Rieckmann K. H., Heidrich H. G. Marker enzymes of Plasmodium falciparum and human erythrocytes as indicators of parasite purity. J Parasitol. 1982 Dec;68(6):1068–1071. [PubMed] [Google Scholar]
- Vincent H. M., Wilson R. J. Malarial antigens on infected erythrocytes. Trans R Soc Trop Med Hyg. 1980;74(4):452–455. doi: 10.1016/0035-9203(80)90052-8. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Wallach D. F., Lin P. S., Hendricks J. Erythrocyte membrane alterations due to infection with Plasmodium berghei. Biochim Biophys Acta. 1973 Nov 16;323(4):539–546. doi: 10.1016/0005-2736(73)90162-4. [DOI] [PubMed] [Google Scholar]
- Williamson P., Algarin L., Bateman J., Choe H. R., Schlegel R. A. Phospholipid asymmetry in human erythrocyte ghosts. J Cell Physiol. 1985 May;123(2):209–214. doi: 10.1002/jcp.1041230209. [DOI] [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- Wiser M. F., Wood P. A., Eaton J. W., Sheppard J. R. Membrane-associated phosphoproteins in Plasmodium berghei-infected murine erythrocytes. J Cell Biol. 1983 Jul;97(1):196–201. doi: 10.1083/jcb.97.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuthavong Y., Wilairat P., Panijpan B., Potiwan C., Beale G. H. Alterations in membrane proteins of mouse erythrocytes infected with different species and strains of malaria parasites. Comp Biochem Physiol B. 1979;63(1):83–85. doi: 10.1016/0305-0491(79)90238-4. [DOI] [PubMed] [Google Scholar]
- Zachowski A., Favre E., Cribier S., Hervé P., Devaux P. F. Outside-inside translocation of aminophospholipids in the human erythrocyte membrane is mediated by a specific enzyme. Biochemistry. 1986 May 6;25(9):2585–2590. doi: 10.1021/bi00357a046. [DOI] [PubMed] [Google Scholar]
- Zachowski A., Fellman P., Devaux P. F. Absence of transbilayer diffusion of spin-labeled sphingomyelin on human erythrocytes. Comparison with the diffusion of several spin-labeled glycerophospholipids. Biochim Biophys Acta. 1985 May 28;815(3):510–514. doi: 10.1016/0005-2736(85)90380-3. [DOI] [PubMed] [Google Scholar]
- van Meer G., Op den Kamp J. A. Transbilayer movement of various phosphatidylcholine species in intact human erythrocytes. J Cell Biochem. 1982;19(2):193–204. doi: 10.1002/jcb.240190209. [DOI] [PubMed] [Google Scholar]




