Abstract
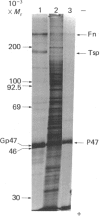
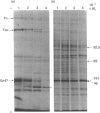
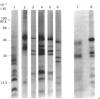
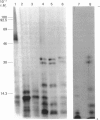
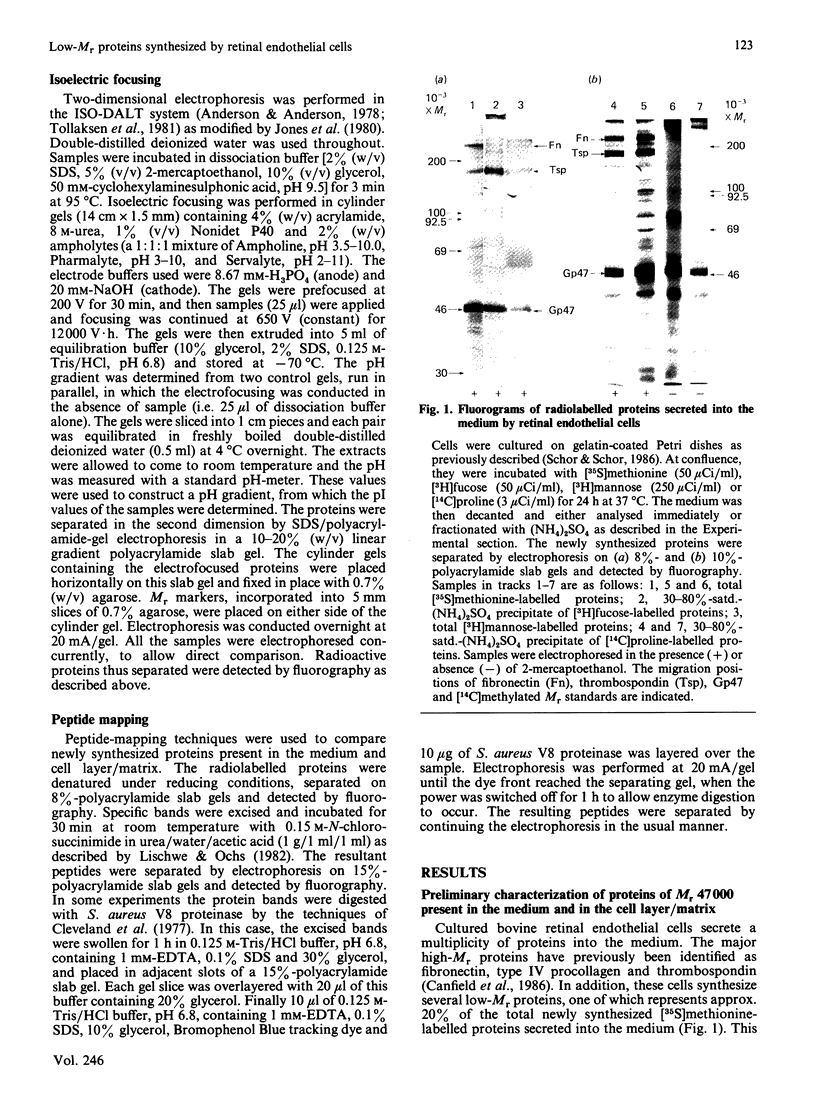
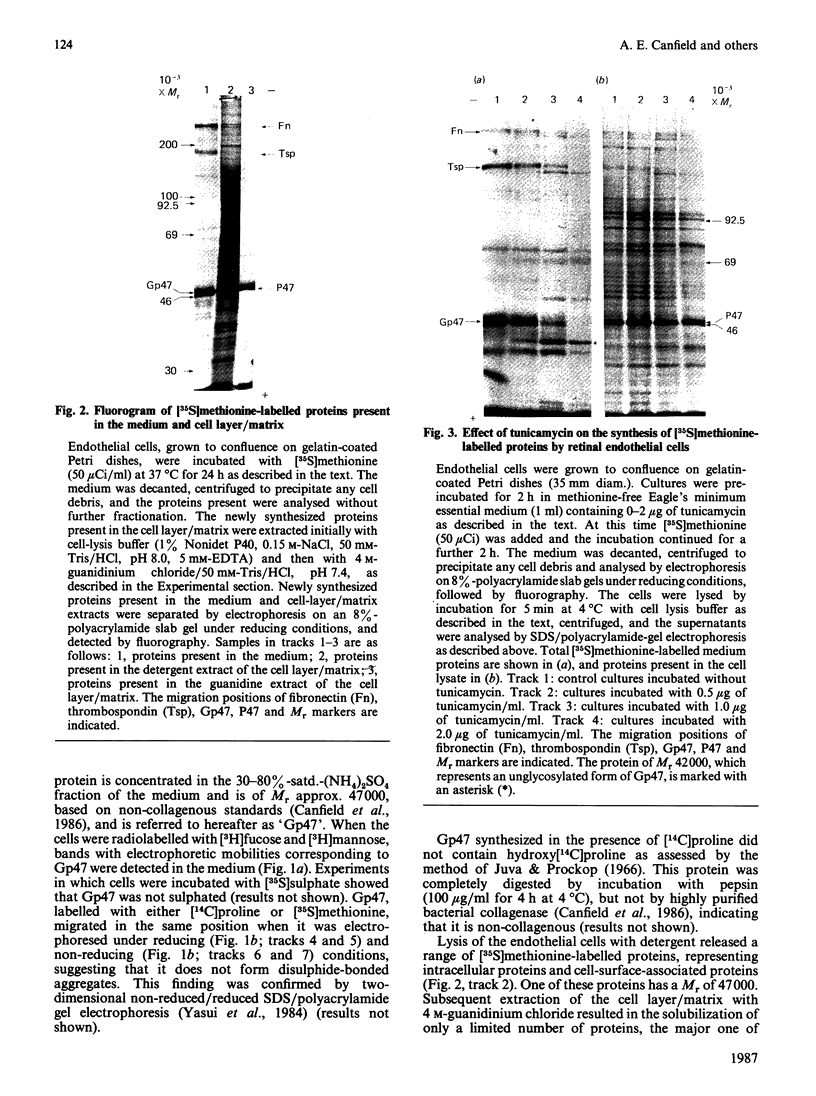
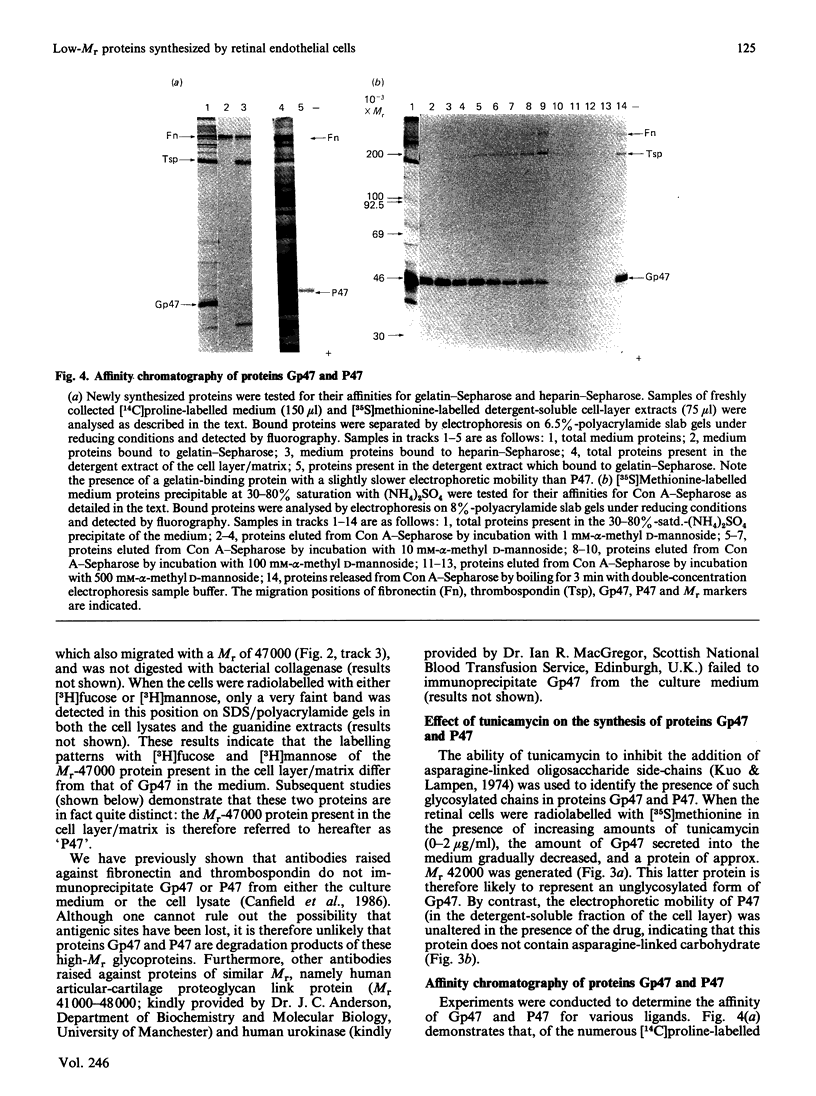
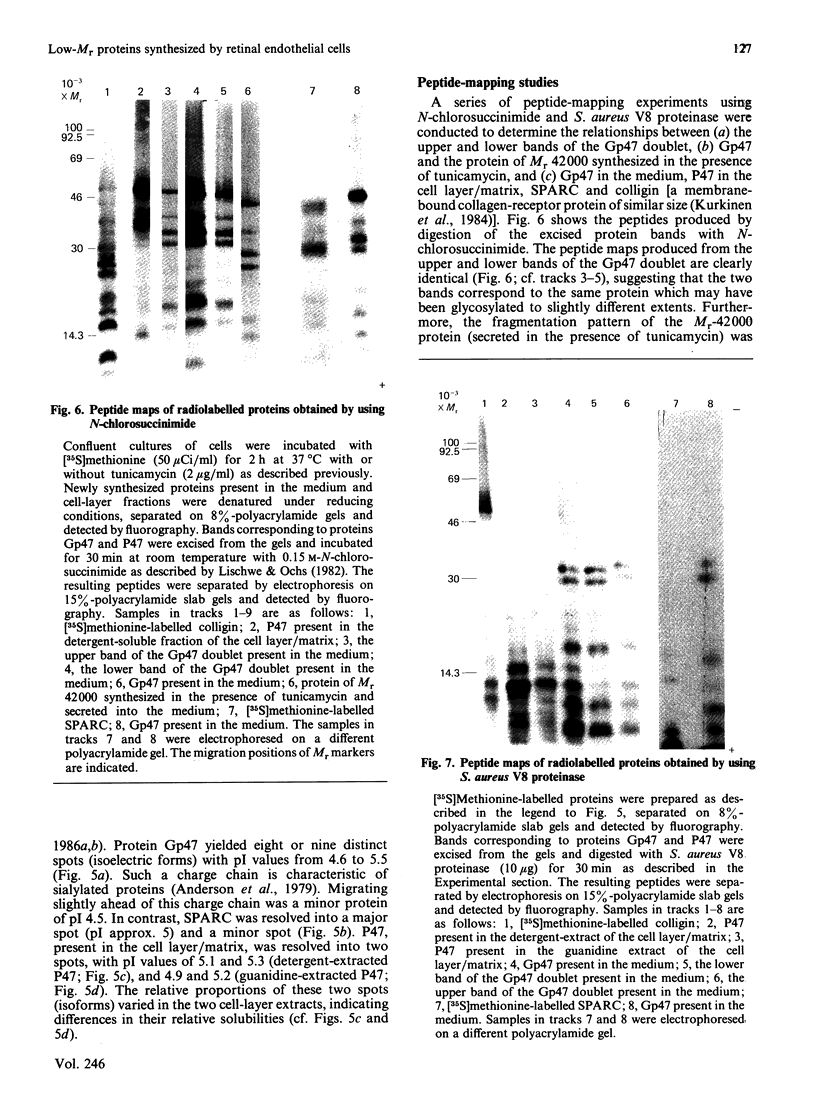
Biosynthetic experiments with cultured bovine retinal endothelial cells have identified a glycoprotein of Mr 47,000 (Gp47) as a major component secreted into the medium. Gp47 is a non-collagenous glycoprotein with a pI of 4.6-5.5, which does not bind to either gelatin-Sepharose or heparin-Sepharose but is retained by concanavalin A-Sepharose. The Mr of this species decreases to approx. 42,000 in the presence of tunicamycin, indicating that it contains asparagine-linked oligosaccharides. A second protein of Mr 47,000 (P47) is present in the cell layer/matrix of these cultured cells. The electrophoretic mobility of P47 remains unaltered when synthesized in the presence of tunicamycin. Peptide-mapping experiments using N-chlorosuccinimide and Staphylococcus aureus V8 proteinase demonstrate that Gp47 and P47 are distinct proteins, and are not related to colligin, a membrane-bound collagen-receptor protein of similar size, or to SPARC, a major secreted product of parietal endodermal cells and sparse cultures of aortic endothelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L., Tollaksen S. L. Proteins of human urine. I. Concentration and analysis by two-dimensional electrophoresis. Clin Chem. 1979 Jul;25(7):1199–1210. [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Osikowicz G., Feder S., Scheinbuks J. Isolation and characterization of a urokinase-type plasminogen activator (Mr = 54,000) from cultured human endothelial cells indistinguishable from urinary urokinase. J Biol Chem. 1984 Jun 10;259(11):7198–7205. [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Schor S. L., Grant M. E. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986 Apr 15;235(2):375–383. doi: 10.1042/bj2350375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Duhamel R. C., Meezan E., Brendel K. Selective solubilization of two populations of polypeptides from bovine retinal basement membranes. Exp Eye Res. 1983 Feb;36(2):257–267. doi: 10.1016/0014-4835(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Heathcote J. G., Orkin R. W. Current concepts of basement-membrane structure and function. Biosci Rep. 1981 Nov;1(11):819–842. doi: 10.1007/BF01114816. [DOI] [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Jaffe E. A., Rifkin D. B. Plasminogen activator and collagenase production by cultured capillary endothelial cells. J Cell Biol. 1982 Dec;95(3):974–981. doi: 10.1083/jcb.95.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Grant M. E. The molecular organization of basement membranes. Int Rev Connect Tissue Res. 1981;9:191–264. doi: 10.1016/b978-0-12-363709-3.50011-5. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Allen D. Biosynthesis of cell surface sulfated glycoproteins by cultured vascular endothelial cells. Biochemistry. 1982 Jan 5;21(1):171–177. doi: 10.1021/bi00530a029. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Watson C., Johnson A. R., Roberts M. K. Sulfated glycoproteins secreted by human vascular endothelial cells. J Biol Chem. 1982 Nov 25;257(22):13581–13586. [PubMed] [Google Scholar]
- Jones M. I., Massingham W. E., Spragg S. P. Electrophoresis tank for running multiple slab gels. Anal Biochem. 1980 Aug;106(2):446–449. doi: 10.1016/0003-2697(80)90546-1. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Taylor A., Garrels J. I., Hogan B. L. Cell surface-associated proteins which bind native type IV collagen or gelatin. J Biol Chem. 1984 May 10;259(9):5915–5922. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Andreasen P. A., Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986 Dec;103(6 Pt 1):2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laug W. E. Secretion of plasminogen activators by cultured bovine endothelial cells: partial purification, characterization and evidence for multiple forms. Thromb Haemost. 1981 Jun 30;45(3):219–224. [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol. 1982 Sep;94(3):631–636. doi: 10.1083/jcb.94.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D. W., 2nd, Braunschweiger K. I., Braunschweiger W. R., Smith J. R. Cell type specific electrophoretic polypeptide profiles of cultured bovine cells. J Cell Sci. 1984 Dec;72:135–145. doi: 10.1242/jcs.72.1.135. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yurchenco P. D., Furthmayr H. The ultrastructural organization and architecture of basement membranes. Ciba Found Symp. 1984;108:6–24. doi: 10.1002/9780470720899.ch2. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Dziadek M., Suchanek C., Huttner W. B., Timpl R. Nature of sulphated macromolecules in mouse Reichert's membrane. Evidence for tyrosine O-sulphate in basement-membrane proteins. Biochem J. 1985 Nov 1;231(3):571–579. doi: 10.1042/bj2310571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Gossage D., Kang A. H. Pretranslational regulation of type I collagen, fibronectin, and a 50-kilodalton noncollagenous extracellular protein by dexamethasone in rat fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4677–4684. [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Tupper J., Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cell Physiol. 1986 Jun;127(3):373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. The isolation and culture of endothelial cells and pericytes from the bovine retinal microvasculature: a comparative study with large vessel vascular cells. Microvasc Res. 1986 Jul;32(1):21–38. doi: 10.1016/0026-2862(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Taylor A., Hogan B. L., Watt F. M. Biosynthesis of EGF receptor, transferrin receptor and colligin by cultured human keratinocytes and the effect of retinoic acid. Exp Cell Res. 1985 Jul;159(1):47–54. doi: 10.1016/s0014-4827(85)80036-7. [DOI] [PubMed] [Google Scholar]
- Yasui N., Benya P. D., Nimni M. E. Identification of a large interrupted helical domain of disulfide-bonded cartilage collagen. J Biol Chem. 1984 Nov 25;259(22):14175–14179. [PubMed] [Google Scholar]
- van Mourik J. A., Lawrence D. A., Loskutoff D. J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem. 1984 Dec 10;259(23):14914–14921. [PubMed] [Google Scholar]