Abstract
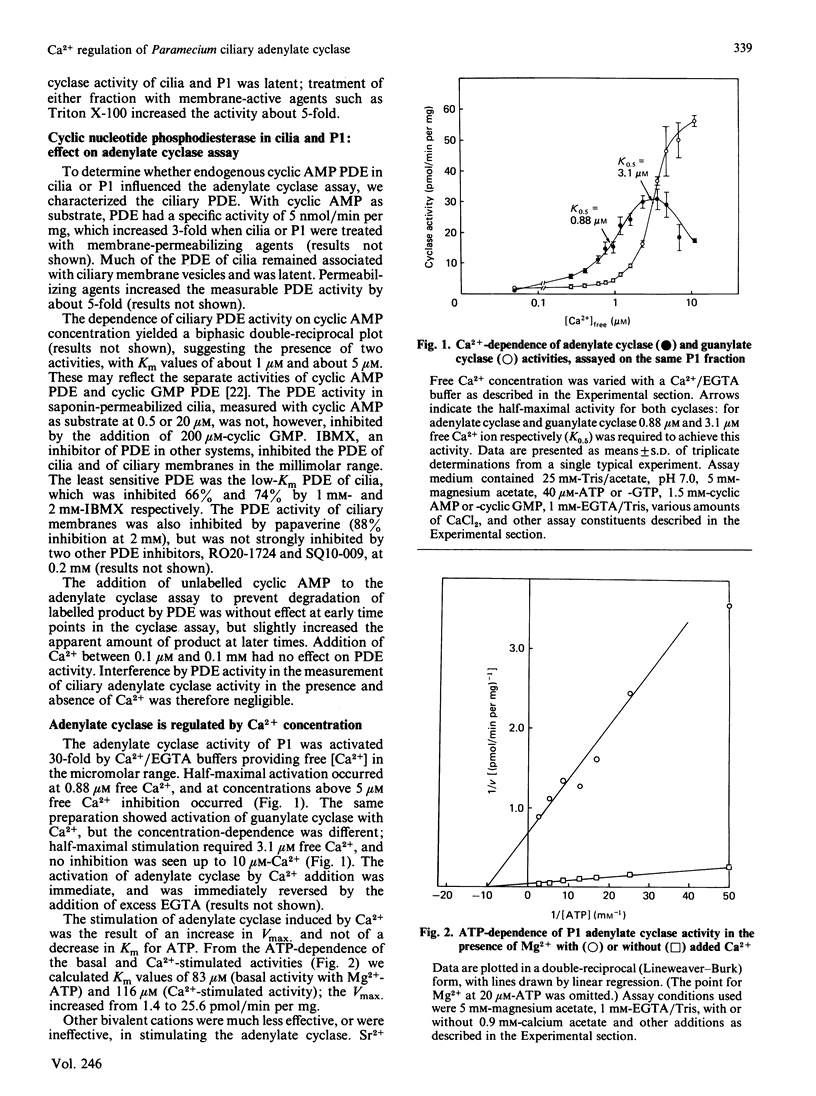
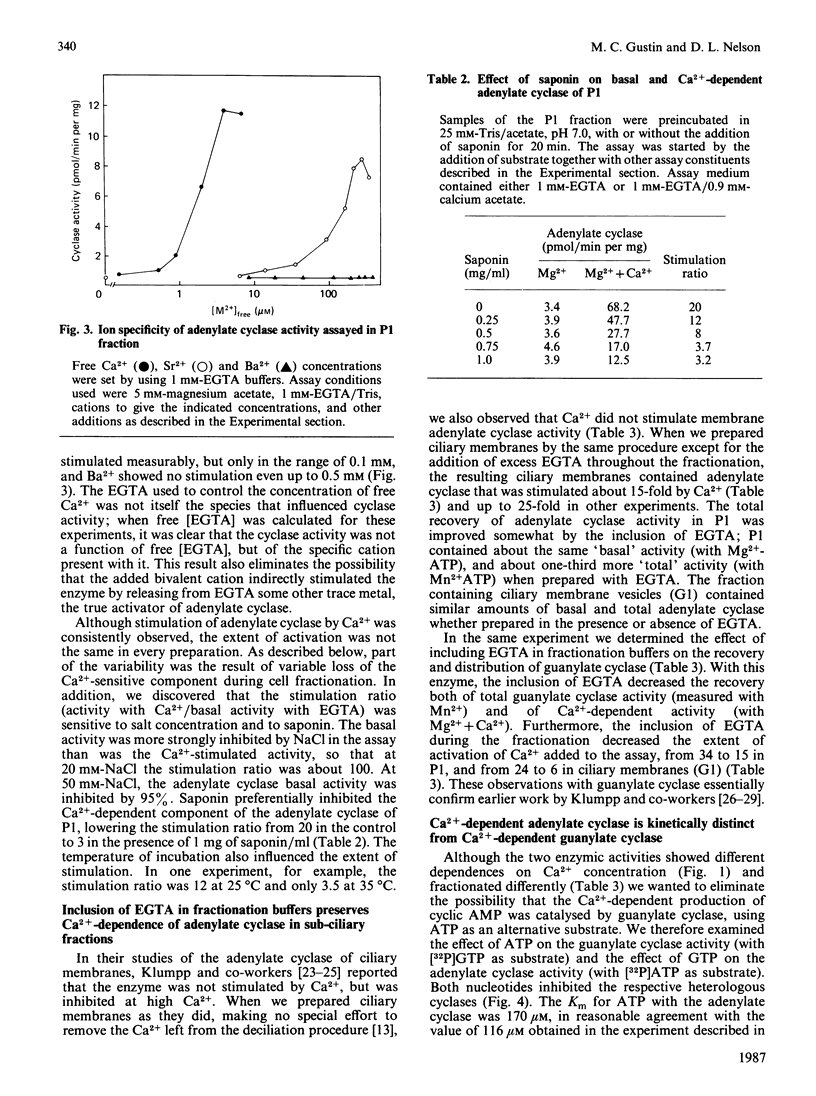
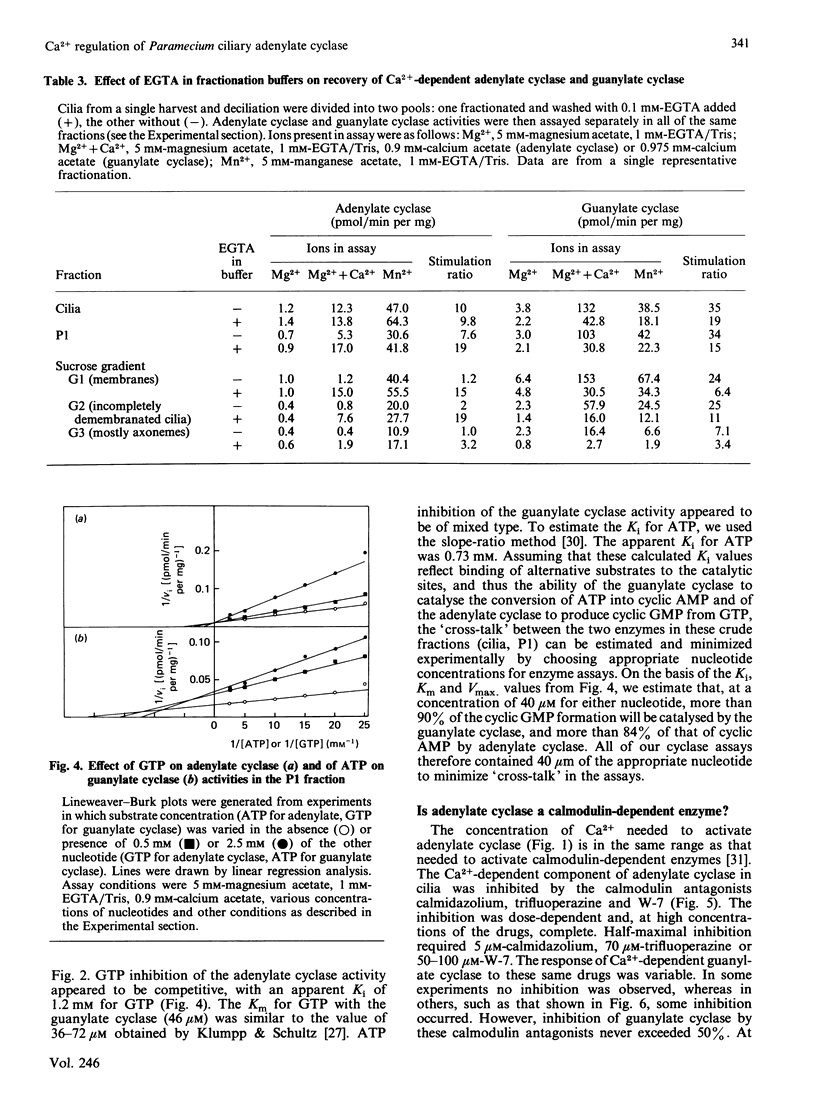
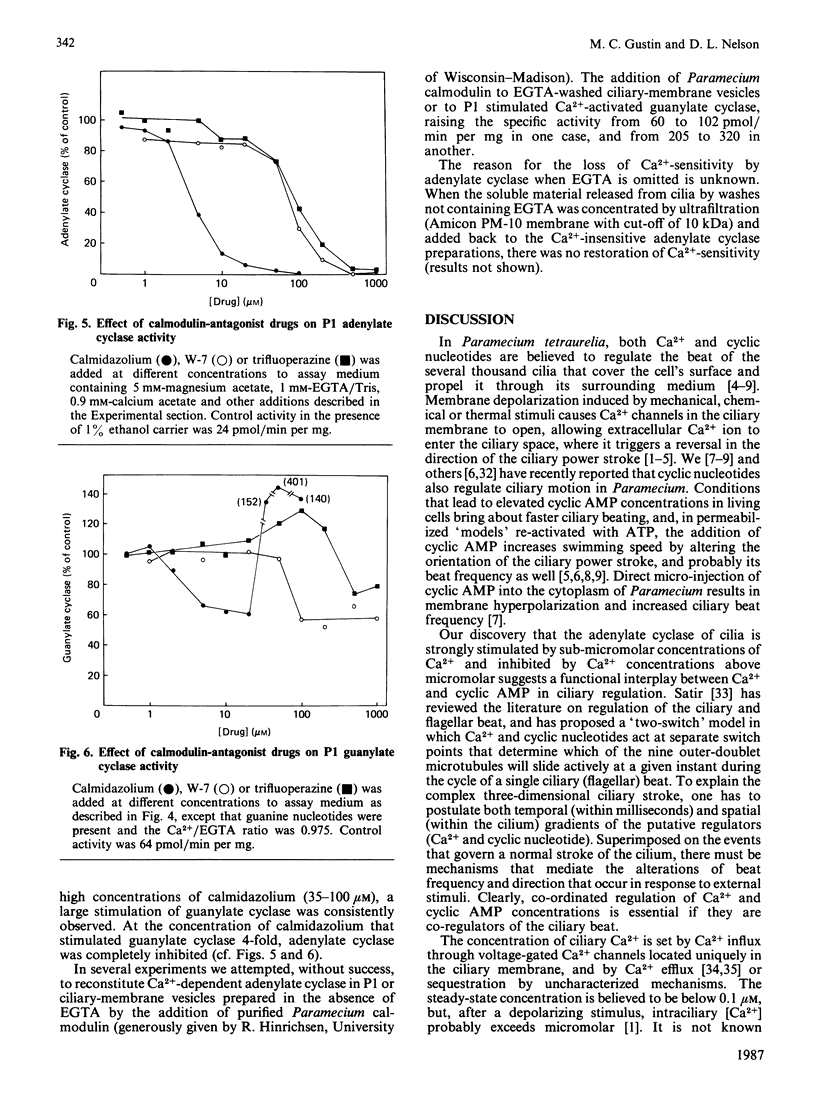
In the ciliated protozoan Paramecium, Ca2+ and cyclic nucleotides are believed to act as second messengers in the regulation of the ciliary beat. Ciliary adenylate cyclase was activated 20-30-fold (half-maximal at 0.8 microM) and inhibited by higher concentrations (10-20 microM) of free Ca2+ ion. Ca2+ activation was the result of an increase in Vmax., not a change in Km for ATP. The activation by Ca2+ was seen only with Mg2+ATP as substrate; with Mn2+ATP the basal adenylate cyclase activity was 10-20-fold above that with Mg2+ATP, and there was no further activation by Ca2+. The stimulation by Ca2+ of the enzyme in cilia and ciliary membranes was blocked by the calmodulin antagonists calmidazolium (half-inhibition at 5 microM), trifluoperazine (70 microM) and W-7 (50-100 microM). When ciliary membranes (which contained most of the ciliary adenylate cyclase) were prepared in the presence of Ca2+, their adenylate cyclase was insensitive to Ca2+ in the assay. However, the inclusion of EGTA in buffers used for fractionation of cilia resulted in full retention of Ca2+-sensitivity by the ciliary membrane adenylate cyclase. The membrane-active agent saponin specifically suppressed the Ca2+-dependent adenylate cyclase without inhibiting basal activity with Mg2+ATP or Mn2+ATP. The ciliary adenylate cyclase was shown to be distinct from the Ca2+-dependent guanylate cyclase; the two activities had different kinetic parameters and different responses to added calmodulin and calmodulin antagonists. Our results suggest that Ca2+ influx through the voltage-sensitive Ca2+ channels in the ciliary membrane may influence intraciliary cyclic AMP concentrations by regulating adenylate cyclase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Ramanathan R., Lewis R. M., Dute R. R., Ling K. Y., Kung C., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium tetraurelia. III. Proteins of cilia and ciliary membranes. J Cell Biol. 1980 Mar;84(3):717–738. doi: 10.1083/jcb.84.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Torres H. N., Flawiá M. M., Fricke R. F. Improved methods for determination of guanylyl cyclase activity and synthesis of [alpha-32P]GTP. Anal Biochem. 1979 Feb;93(1):124–133. [PubMed] [Google Scholar]
- Bonini N. M., Gustin M. C., Nelson D. L. Regulation of ciliary motility by membrane potential in Paramecium: a role for cyclic AMP. Cell Motil Cytoskeleton. 1986;6(3):256–272. doi: 10.1002/cm.970060303. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium aurelia. I. 45Ca2+ fluxes across resting and excited membrane. Biochim Biophys Acta. 1976 Oct 5;448(2):338–351. doi: 10.1016/0005-2736(76)90247-9. [DOI] [PubMed] [Google Scholar]
- Da Silveira J. F., Zingales B., Colli W. Characterization of an adenylyl cyclase activity in particulate preparations from epimastigote forms of Trypanosoma cruzi. Biochim Biophys Acta. 1977 Apr 12;481(2):722–733. doi: 10.1016/0005-2744(77)90306-0. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eisenschlos C., Flawiá M. M., Torruella M., Torres H. N. Interaction of Trypanosoma cruzi adenylate cyclase with liver regulatory factors. Biochem J. 1986 May 15;236(1):185–191. doi: 10.1042/bj2360185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L., Kopf G. S. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res. 1980;13:251–306. [PubMed] [Google Scholar]
- Garbers D. L., Murad F. Guanylate cyclase assay methods. Adv Cyclic Nucleotide Res. 1979;10:57–67. [PubMed] [Google Scholar]
- Hennessey T., Machemer H., Nelson D. L. Injected cyclic AMP increases ciliary beat frequency in conjunction with membrane hyperpolarization. Eur J Cell Biol. 1985 Mar;36(2):153–156. [PubMed] [Google Scholar]
- Hinrichsen R. D., Burgess-Cassler A., Soltvedt B. C., Hennessey T., Kung C. Restoration by calmodulin of a Ca2+-dependent K+ current missing in a mutant of Paramecium. Science. 1986 Apr 25;232(4749):503–506. doi: 10.1126/science.2421410. [DOI] [PubMed] [Google Scholar]
- Klumpp S., Kleefeld G., Schultz J. E. Calcium/calmodulin-regulated guanylate cyclase of the excitable ciliary membrane from Paramecium. Dissociation of calmodulin by La3+: calmodulin specificity and properties of the reconstituted guanylate cyclase. J Biol Chem. 1983 Oct 25;258(20):12455–12459. [PubMed] [Google Scholar]
- Klumpp S., Schultz J. E. Characterization of a Ca2+-dependent guanylate cyclase in the excitable ciliary membrane from Paramecium. Eur J Biochem. 1982 May 17;124(2):317–324. doi: 10.1111/j.1432-1033.1982.tb06594.x. [DOI] [PubMed] [Google Scholar]
- Klumpp S., Steiner A. L., Schultz J. E. Immunocytochemical localization of cyclic GMP, cGMP-dependent protein kinase, calmodulin and calcineurin in Paramecium tetraurelia. Eur J Cell Biol. 1983 Nov;32(1):164–170. [PubMed] [Google Scholar]
- Kopf G. S., Vacquier V. D. Characterization of a calcium-modulated adenylate cyclase from abalone spermatozoa. Biol Reprod. 1985 Dec;33(5):1094–1104. doi: 10.1095/biolreprod33.5.1094. [DOI] [PubMed] [Google Scholar]
- Kopf G. S., Vacquier V. D. Characterization of a calmodulin-stimulated adenylate cyclase from abalone spermatozoa. J Biol Chem. 1984 Jun 25;259(12):7590–7596. [PubMed] [Google Scholar]
- Kopf G. S., Woolkalis M. J., Gerton G. L. Evidence for a guanine nucleotide-binding regulatory protein in invertebrate and mammalian sperm. Identification by islet-activating protein-catalyzed ADP-ribosylation and immunochemical methods. J Biol Chem. 1986 Jun 5;261(16):7327–7331. [PubMed] [Google Scholar]
- Kudo S., Muto Y., Nozawa Y. Regulation by calcium of hormone-insensitive adenylate cyclase and calmodulin-dependent guanylate cyclase in Tetrahymena plasma membrane. Comp Biochem Physiol B. 1985;80(4):813–816. doi: 10.1016/0305-0491(85)90466-3. [DOI] [PubMed] [Google Scholar]
- Kung C., Saimi Y. The physiological basis of taxes in Paramecium. Annu Rev Physiol. 1982;44:519–534. doi: 10.1146/annurev.ph.44.030182.002511. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacNeil S., Lakey T., Tomlinson S. Calmodulin regulation of adenylate cyclase activity. Cell Calcium. 1985 Jun;6(3):213–216. doi: 10.1016/0143-4160(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Martinac B., Hildebrand E. Electrically induced Ca2+ transport across the membrane of Paramecium caudatum measured by means of flow-through technique. Biochim Biophys Acta. 1981 Dec 7;649(2):244–252. doi: 10.1016/0005-2736(81)90412-0. [DOI] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Nagao S., Kudo S., Nozawa Y. Effects of phenothiazines on the membrane-bound guanylate and adenylate cyclase in Tetrahymena pyriformis. Biochem Pharmacol. 1981 Oct 1;30(19):2709–2712. doi: 10.1016/0006-2952(81)90542-6. [DOI] [PubMed] [Google Scholar]
- Nagao S., Kudo S., Nozawa Y. Inhibitory effects of calmodulin antagonists on plasma membrane cyclases in Tetrahymena: calmodulin-dependent guanylate cyclase and calmodulin-independent adenylate cyclase. Biochem Pharmacol. 1983 Sep 1;32(17):2501–2504. doi: 10.1016/0006-2952(83)90009-6. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Control of ciliary activities by adenosinetriphosphate and divalent cations in triton-extracted models of Paramecium caudatum. J Exp Biol. 1973 Jun;58(3):657–676. doi: 10.1242/jeb.58.3.657. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Ooi H. Regulation of ciliary reversal in triton-extracted Paramecium by calcium and cyclic adenosine monophosphate. J Cell Sci. 1985 Aug;77:185–195. doi: 10.1242/jcs.77.1.185. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Tanaka H., Oosawa F. Ca2+-dependent regulation of beat frequency of cilia in Paramecium. J Cell Sci. 1984 Jan;65:223–231. doi: 10.1242/jcs.65.1.223. [DOI] [PubMed] [Google Scholar]
- Ogura A., Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature. 1976 Nov 11;264(5582):170–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- Reig J. A., Kornblihtt A. R., Flawiá M. M., Torres H. N. Soluble adenylate cyclase activity in Neurospora crassa. Biochem J. 1982 Oct 1;207(1):43–49. doi: 10.1042/bj2070043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig J. A., Téllez-Iñn M. T., Flawiá M. M., Torres H. N. Activation of Neurospora crassa soluble adenylate cyclase by calmodulin. Biochem J. 1984 Jul 15;221(2):541–543. doi: 10.1042/bj2210541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Grünemund R., von Hirschhausen R., Schönefeld U. Ionic regulation of cyclic AMP levels in Paramecium tetraurelia in vivo. FEBS Lett. 1984 Feb 13;167(1):113–116. doi: 10.1016/0014-5793(84)80843-1. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Klumpp S. Guanylate cyclase in the excitable ciliary membrane of Paramecium. FEBS Lett. 1980 Dec 15;122(1):64–66. doi: 10.1016/0014-5793(80)80402-9. [DOI] [PubMed] [Google Scholar]
- Sinha A. K., Colman R. W. A new method for separating cyclic AMP from 5'-AMP with application to the assay for cyclic AMP phosphodiesterase. Anal Biochem. 1981 May 15;113(2):239–245. doi: 10.1016/0003-2697(81)90072-5. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983 Feb;28(1):75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- Torruella M., Flawiá M. M., Eisenschlos C., Molina y Vedia L., Rubinstein C. P., Torres H. N. Trypanosoma cruzi adenylate cyclase activity. Purification and characterization. Biochem J. 1986 Feb 15;234(1):145–150. doi: 10.1042/bj2340145. [DOI] [PMC free article] [PubMed] [Google Scholar]


