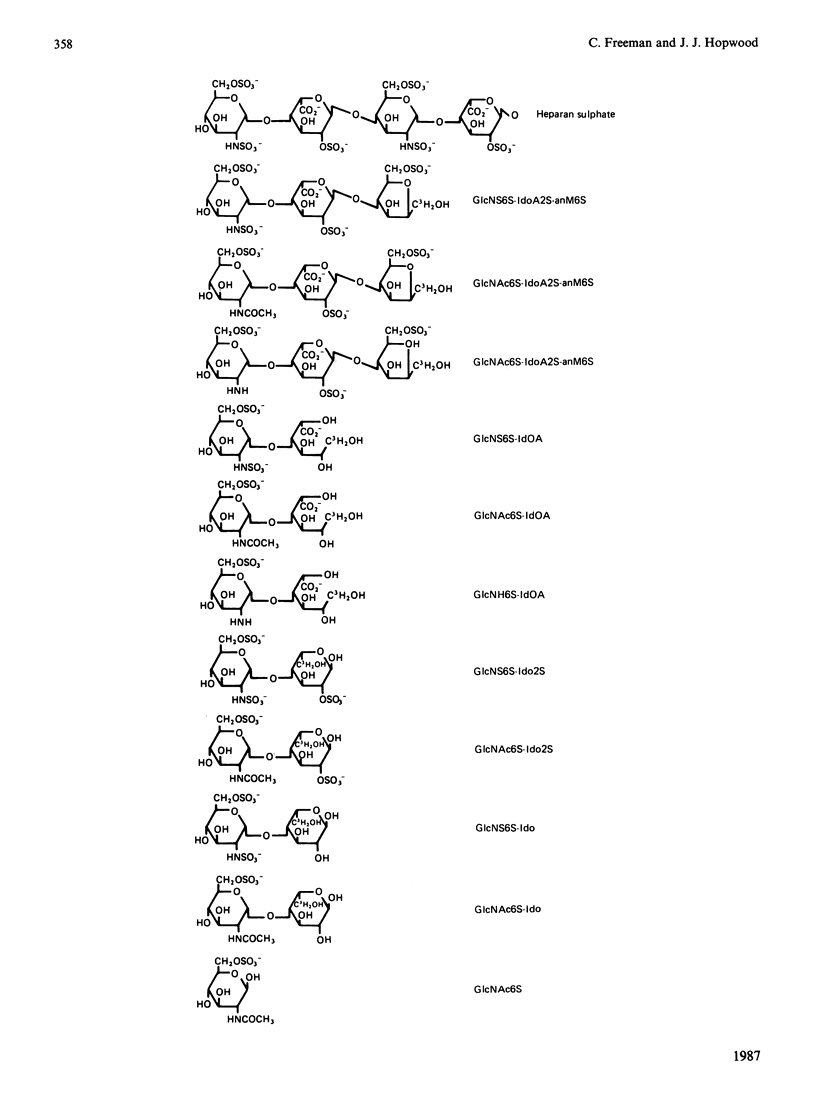
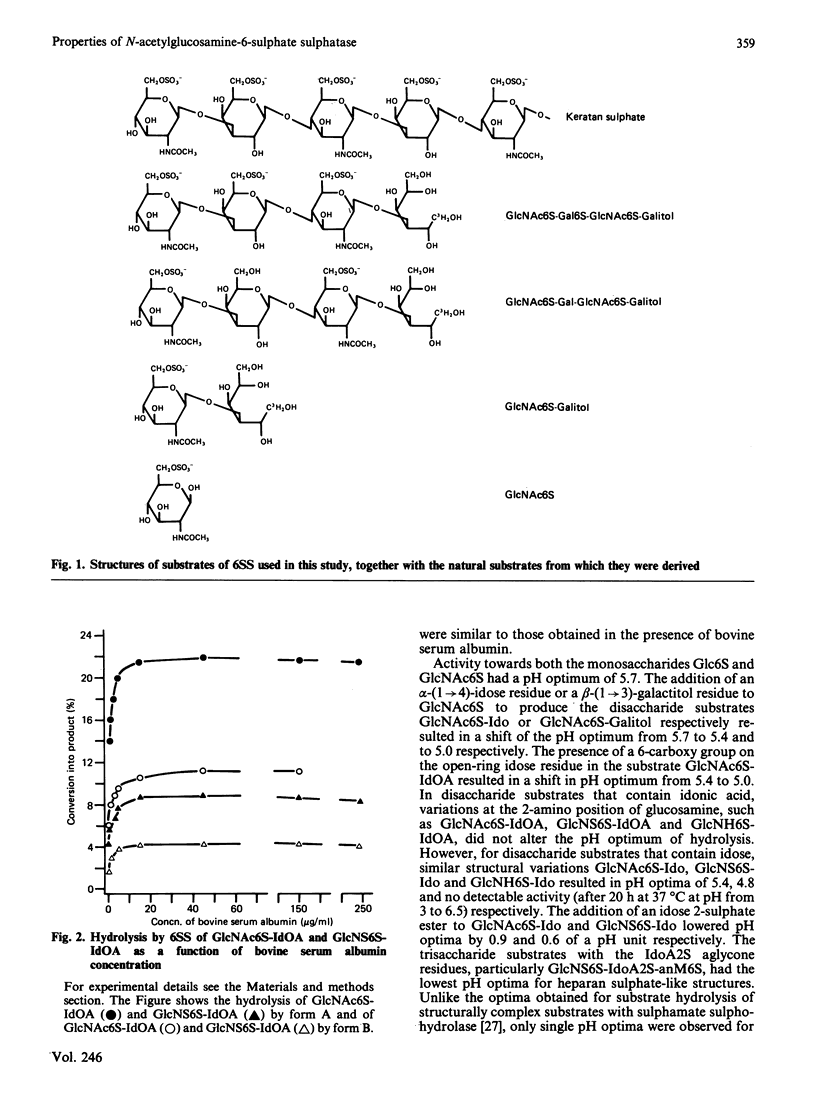
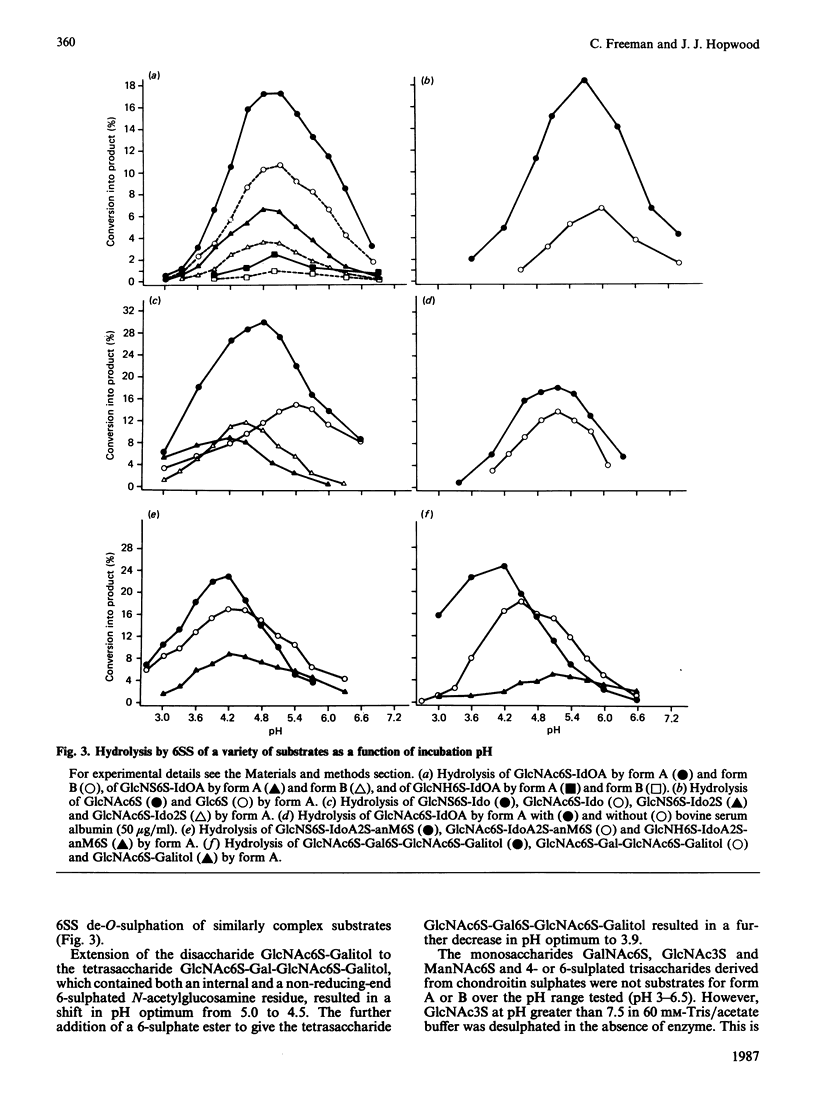
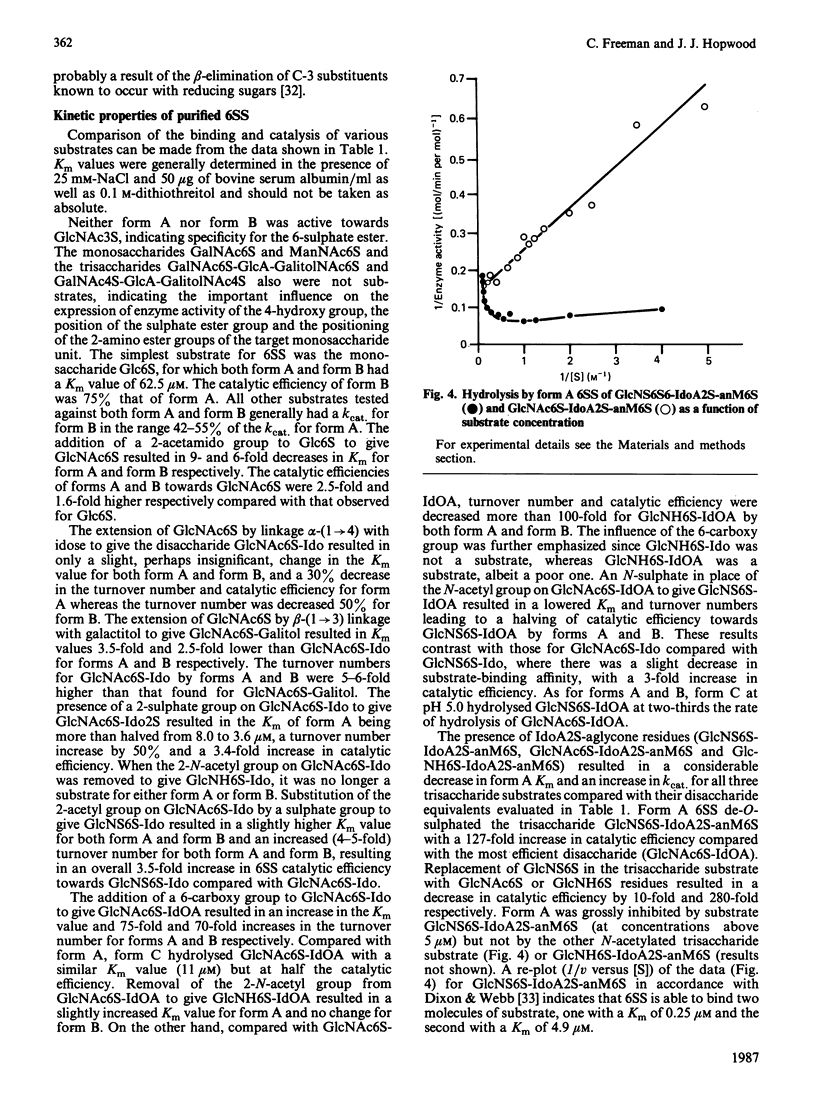
Abstract
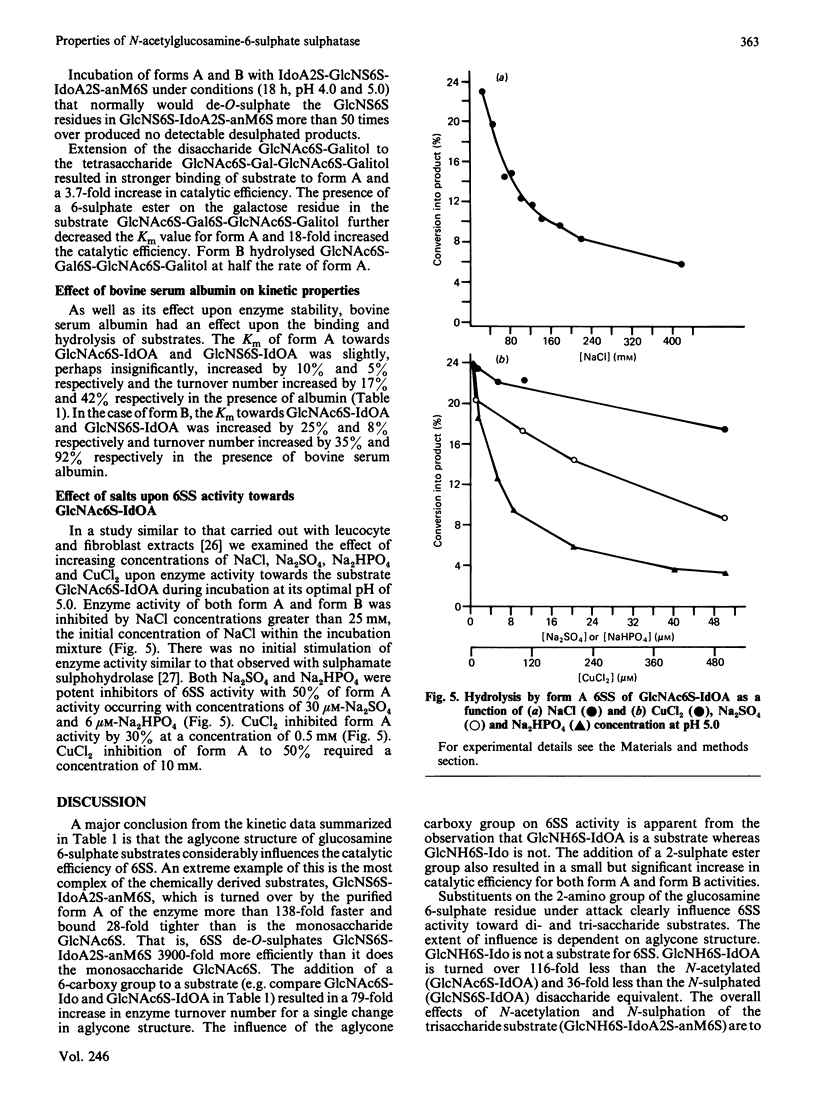
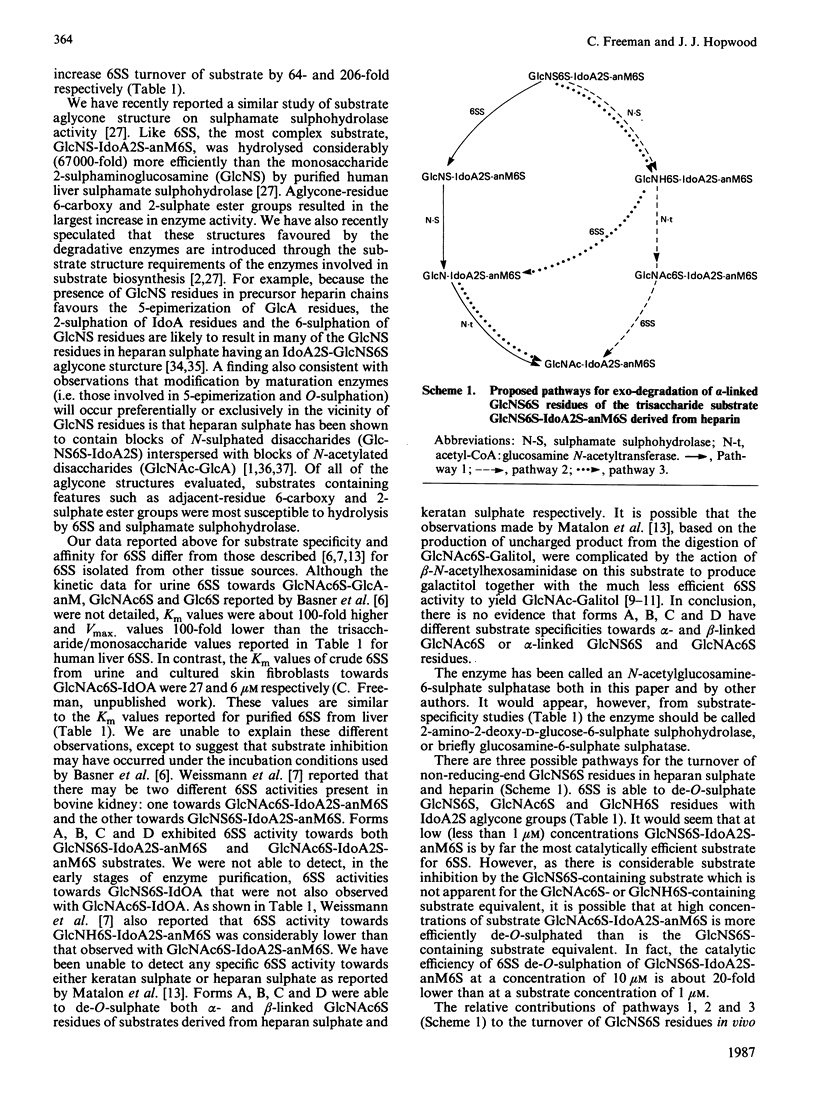
Kinetic parameters (Km and kcat.) of the two major forms (A and B) and a minor form (C) of human liver N-acetylglucosamine-6-sulphate sulphatase [Freeman, Clements & Hopwood (1987) Biochem. J. 246, 347-354] were determined with a variety of substrates matching structural aspects of the physiological substrates in vivo, namely heparin, heparan sulphate and keratan sulphate. Enzyme activity is highly specific towards glucosamine 6-sulphate or glucose 6-sulphate residues. More structurally complex substrates, in which several aspects of the aglycone structure of the natural substrate were maintained, are hydrolysed with catalytic efficiencies up to 3900 times above that observed for the monosaccharide substrate N-acetylglucosamine 6-sulphate. Forms A and B both desulphate substrates derived from keratan sulphate and heparin. Aglycone structures that influence substrate binding and/or enzyme activity were penultimate-residue 6-carboxy and 2-sulphate ester groups for heparin-derived substrates and penultimate-residue 6-sulphate ester groups for keratan sulphate-derived substrates. The 4-hydroxy group of the N-acetylglucosamine 6-sulphate or the 2-sulphaminoglucosamine 6-sulphate under enzymic attack is involved in the catalytic mechanism. The presence of a 2-amino group in place of a 2-acetamido or a 2-sulphoamino group considerably decreases the catalytic efficiency of the sulphatase, particularly in the absence of a penultimate-aglycone-residue 6-carboxy group. Both forms A and B are exo-enzymes, since activity towards internal sulphate ester bonds was not observed. The effect of incubation pH on enzyme activity towards the variety of substrates evaluated was complex and dependent on substrate aglycone structure. The presence of aglycone 2-sulphate ester, 6-carboxy group and 6-sulphate ester groups on the glucosamine 6-sulphate residue under attack considerably affects the pH response. Sulphate and phosphate ions are potent inhibitors of enzyme activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basner R., Kresse H., von Figura K. N-Acetylglucosamine-6-sulfate sulfatase from human urine. J Biol Chem. 1979 Feb 25;254(4):1151–1158. [PubMed] [Google Scholar]
- Brown W. E., Seamon K. B. Quantitation and characterization of the trifluoroacetonyl derivative of cysteine: a useful NMR probe. Anal Biochem. 1978 Jun 15;87(1):211–222. doi: 10.1016/0003-2697(78)90587-0. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Elliott H., Hopwood J. J. Detection of the Sanfilippo D syndrome by the use of a radiolabeled monosaccharide sulfate as the substrate for the estimation of N-acetylglucosamine-6-sulfate sulfatase. Anal Biochem. 1984 Apr;138(1):205–209. doi: 10.1016/0003-2697(84)90789-9. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Sjöberg I., Havsmark B. Structural studies on heparan sulphates. Characterization of oligosaccharides; obtained by periodate oxidation and alkaline elimination. Eur J Biochem. 1980 May;106(1):59–69. [PubMed] [Google Scholar]
- Freeman C., Clements P. R., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Purification and characterization. Biochem J. 1987 Sep 1;246(2):347–354. doi: 10.1042/bj2460347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver sulphamate sulphohydrolase. Determinations of native protein and subunit Mr values and influence of substrate agylcone structure on catalytic properties. Biochem J. 1986 Feb 15;234(1):83–92. doi: 10.1042/bj2340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W., Beck M., Kresse H. Intralysosomal formation and metabolic fate of N-acetylglucosamine 6-sulfate from keratan sulfate. Eur J Biochem. 1985 Sep 16;151(3):551–556. doi: 10.1111/j.1432-1033.1985.tb09138.x. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H., Tsuji M., Nakanishi Y., Suzuki S. Separation and properties of five glycosaminoglycan sulfatases from rat skin. J Biol Chem. 1979 Aug 25;254(16):7570–7578. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Detection of Morquio A syndrome using radiolabelled substrates derived from keratan sulphate for the estimation of galactose 6-sulphate sulphatase. Clin Sci (Lond) 1983 Sep;65(3):325–331. doi: 10.1042/cs0650325. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Detection of the Sanfilippo type B syndrome using radiolabelled oligosaccharides as substrates for the estimation of alpha-N-acetylglucosaminidase. Clin Chim Acta. 1982 Mar 26;120(1):77–86. doi: 10.1016/0009-8981(82)90079-1. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Diagnosis of Sanfilippo type A syndrome by estimation of sulfamidase activity using a radiolabelled tetrasaccharide substrate. Clin Chim Acta. 1982 Aug 18;123(3):241–250. doi: 10.1016/0009-8981(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Isolation and characterization of N-acetylglucosamine 6-sulfate from the urine of a patient with Sanfilippo type D syndrome and its occurrence in normal urine. Biochem Int. 1983 Jun;6(6):831–836. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H., Muller V. J., Saccone G. T. Diagnosis of Maroteaux-Lamy syndrome by the use of radiolabelled oligosaccharides as substrates for the determination of arylsulphatase B activity. Biochem J. 1986 Mar 15;234(3):507–514. doi: 10.1042/bj2340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. N-acetylglucosamine 6-sulfate residues in keratan sulfate and heparan sulfate are desulfated by the same enzyme. Biochem Int. 1983 Feb;6(2):141–148. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Radiolabelled oligosaccharides as substrates for the estimation of sulfamidase and the detection of the Sanfilippo type A syndrome. Clin Chim Acta. 1981 Apr 27;112(1):55–66. doi: 10.1016/0009-8981(81)90268-0. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of heparin to produce radio-labelled substrates for sulfamidase, 2-acetamido-2-deoxy-alpha-D-glucosidase, acetyl-CoA:2-amino-2-deoxy-alpha-D-glucoside N-acetyltransferase, and 2-acetamido-2-deoxy-D-glucose 6-sulfate sulfatase. Carbohydr Res. 1981 May 1;91(2):165–190. doi: 10.1016/s0008-6215(00)86029-2. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of keratan sulfate: production of radiolabelled substrates for 6-O-sulfogalactose sulfatase and beta-D-galactosidase. Carbohydr Res. 1983 Jun 16;117:263–274. doi: 10.1016/0008-6215(83)88092-6. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Sulphamidase activity in leucocytes, cultured skin fibroblasts and amniotic cells: diagnosis of the Sanfilippo A syndrome with the use of radiolabelled disaccharide substrate. Clin Sci (Lond) 1981 Dec;61(6):729–735. doi: 10.1042/cs0610729. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. The diagnosis of the Sanfilippo C syndrome, using monosaccharide and oligosaccharide substrates to assay acetyl-CoA: 2-amino-2-deoxy-alpha-glucoside N-acetyltransferase activity. Clin Chim Acta. 1981 Apr 27;112(1):67–75. doi: 10.1016/0009-8981(81)90269-2. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Urinary excretion of sulphated N-acetylhexosamines in patients with various mucopolysaccharidoses. Biochem J. 1985 Aug 1;229(3):579–586. doi: 10.1042/bj2290579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. J. Selective depolymerisation of dermatan sulfate: production of radiolabelled substrates for alpha-L-iduronidase, sulfoiduronate sulfatase, and beta-D-glucuronidase. Carbohydr Res. 1983 Oct 28;122(2):227–239. doi: 10.1016/0008-6215(83)88334-7. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. Diagnostic enzymology of alpha-L-iduronidase with special reference to a sulphated disaccharide derived from heparin. Clin Sci (Lond) 1982 Feb;62(2):193–201. doi: 10.1042/cs0620193. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V., Smithson A., Baggett N. A fluorometric assay using 4-methylumbelliferyl alpha-L-iduronide for the estimation of alpha-L-iduronidase activity and the detection of Hurler and Scheie syndromes. Clin Chim Acta. 1979 Mar 1;92(2):257–265. doi: 10.1016/0009-8981(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The alkali-labile linkage between keratan sulphate and protein. Biochem J. 1974 Jul;141(1):57–69. doi: 10.1042/bj1410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J. alpha-L-iduronidase, beta-D-glucuronidase, and 2-sulfo-L-iduronate 2-sulfatase: preparation and characterization of radioactive substrates from heparin. Carbohydr Res. 1979 Mar;69:203–216. doi: 10.1016/s0008-6215(00)85765-1. [DOI] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U., Jensen J. W., Rodén L., Prihar H., Feingold D. S. Biosynthesis of heparin. Substrate specificity of heparosan N-sulfate D-glucuronosyl 5-epimerase. J Biol Chem. 1984 Jan 25;259(2):1056–1063. [PubMed] [Google Scholar]
- Kresse H., Paschke E., von Figura K., Gilberg W., Fuchs W. Sanfilippo disease type D: deficiency of N-acetylglucosamine-6-sulfate sulfatase required for heparan sulfate degradation. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6822–6826. doi: 10.1073/pnas.77.11.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Wappner R., Deanching M., Brandt I. K., Horwitz A. Keratan and herparan sulfaturia: glucosamine-6-sulfate deficiency. Ann Clin Lab Sci. 1982 May-Jun;12(3):234–238. [PubMed] [Google Scholar]
- Muller V. J., Hopwood J. J. Radiolabelled disaccharides for the assay of beta-D-glucuronidase activity and the detection of mucopolysaccharidosis type VII. Clin Chim Acta. 1982 Aug 18;123(3):357–360. doi: 10.1016/0009-8981(82)90182-6. [DOI] [PubMed] [Google Scholar]
- Weissmann B., Chao H., Chow P. A glucosamine O,N-disulfate O-sulfohydrolase with a probable role in mammalian catabolism of heparan sulfate. Biochem Biophys Res Commun. 1980 Nov 28;97(2):827–833. doi: 10.1016/0006-291x(80)90338-1. [DOI] [PubMed] [Google Scholar]


