Abstract
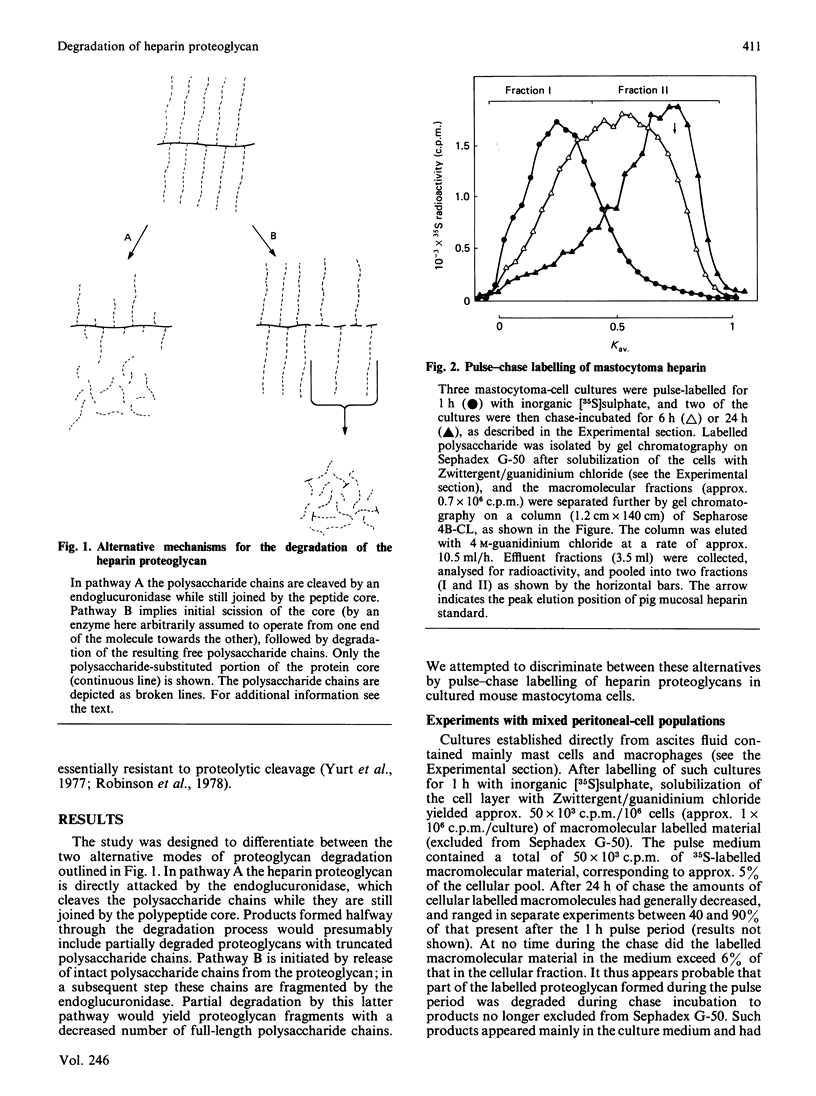
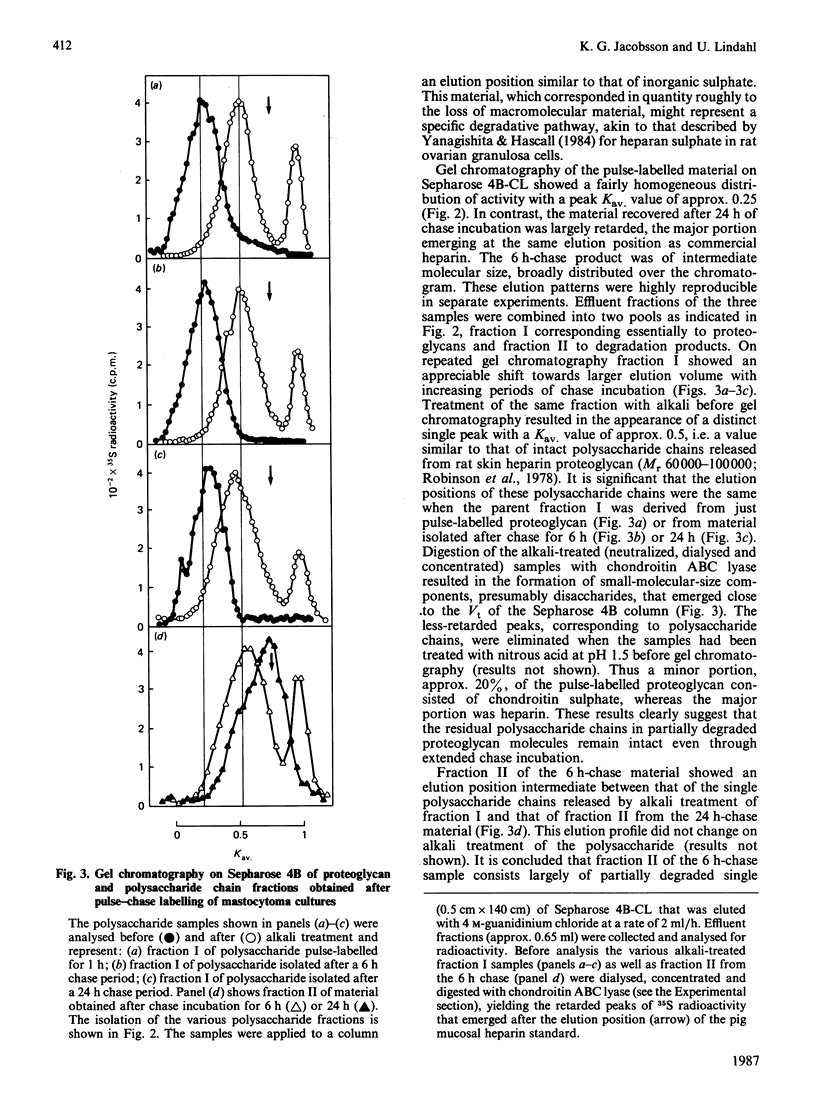
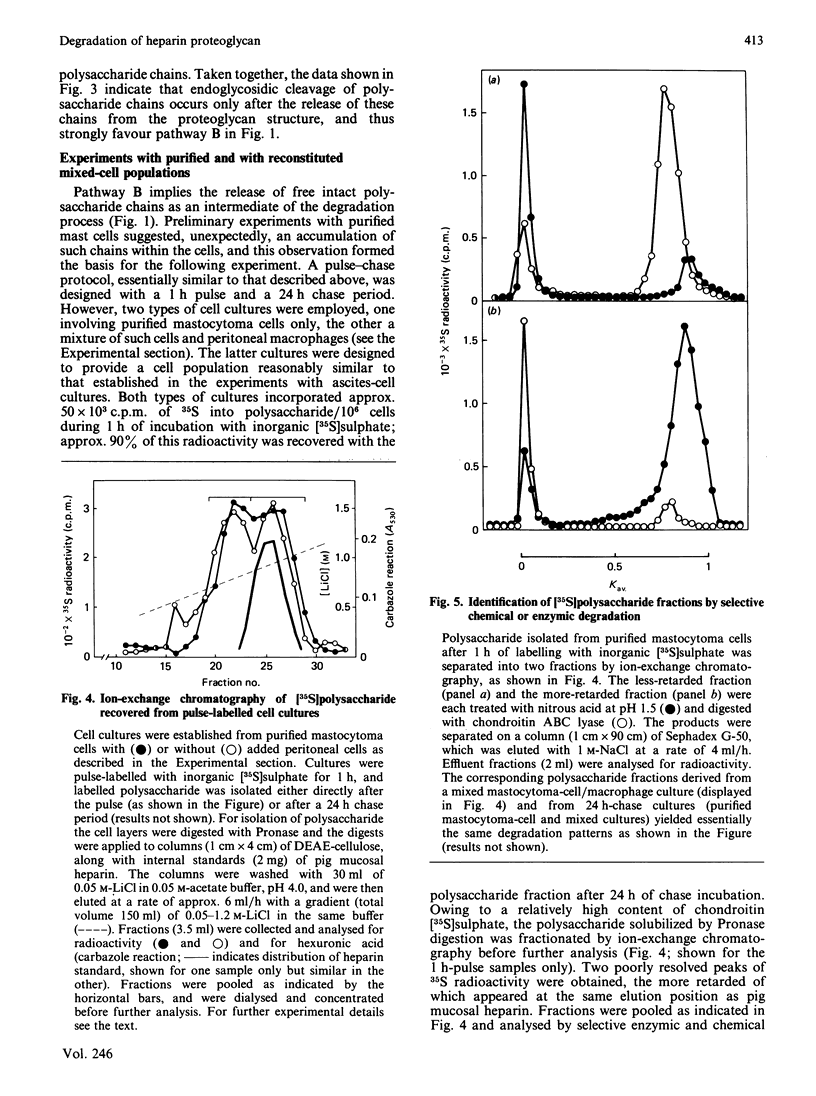
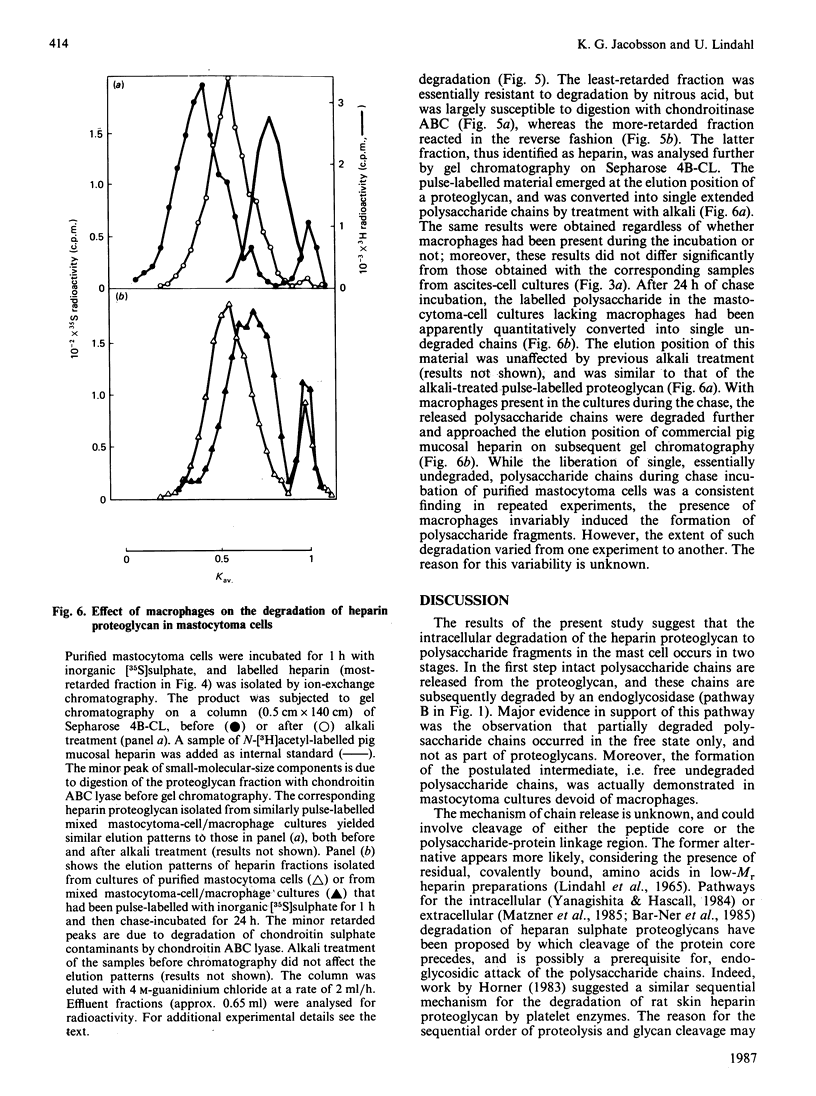
Pulse-labelling of mouse mastocytoma cell cultures, established from ascites fluid, with inorganic [35S]sulphate for 1 h yielded labelled heparin proteoglycan containing polysaccharide chains of Mr 60,000-100,000. After chase incubation for 24 h most of the 35S appeared in intracellular polysaccharide fragments similar in size to commercially available heparin, Mr 5000-25,000, as indicated by gel chromatography. Products isolated from cultures after 6 h of chase incubation consisted of partially degraded free polysaccharide chains and, in addition, residual proteoglycans that were of smaller size than the proteoglycans initially pulse-labelled. The polysaccharide chains released by alkali treatment from the residual chase-incubated proteoglycans were of the same size as the chains derived from proteoglycans after 1 h of pulse labelling. These results suggest that the intracellular degradation of heparin proteoglycan to polysaccharide fragments is initiated by release of intact polysaccharide chains, probably by action of a peptidase, and is pursued through cleavage of these chains by an endoglycosidase. An endoglucuronidase with stringent substrate specificity [Thunberg, Bäckström, Wasteson, Ogren & Lindahl (1982) J. Biol. Chem. 257, 10278-10282] has previously been implicated in the latter step. Cultures of more purified mastocytoma cells (essentially devoid of macrophages) did not metabolize [35S]heparin proteoglycan to polysaccharide fragments, but instead accumulated free intact polysaccharide chains, i.e. the postulated intermediate of the complete degradation pathway. When such purified cells were co-cultured with adherent mouse peritoneal cells, presumably macrophages, formation of polysaccharide fragments was observed. It is tentatively proposed that the expression of endoglucuronidase activity by the mast cells depends on collaboration between these cells and macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Lormeau J. C., Petitou M., Rosenberg R. D., Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985 Nov 5;24(23):6723–6729. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M., Kramer M. D., Schirrmacher V., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Sequential degradation of heparan sulfate in the subendothelial extracellular matrix by highly metastatic lymphoma cells. Int J Cancer. 1985 Apr 15;35(4):483–491. doi: 10.1002/ijc.2910350411. [DOI] [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Macromolecular heparin from rat skin. Isolation, characterization, and depolymerization with ascorbate. J Biol Chem. 1971 Jan 10;246(1):231–239. [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jacobsson K. G., Lindahl U., Horner A. A. Location of antithrombin-binding regions in rat skin heparin proteoglycans. Biochem J. 1986 Dec 15;240(3):625–632. doi: 10.1042/bj2400625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson K. G., Riesenfeld J., Lindahl U. Biosynthesis of heparin. Effects of n-butyrate on cultured mast cells. J Biol Chem. 1985 Oct 5;260(22):12154–12159. [PubMed] [Google Scholar]
- Kimura J. H., Caputo C. B., Hascall V. C. The effect of cycloheximide on synthesis of proteoglycans by cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 May 10;256(9):4368–4376. [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Matzner Y., Bar-Ner M., Yahalom J., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985 Oct;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara A., Jenne B. M., Dean M. F. Fibroblasts acquire beta-glucuronidase by direct and indirect transfer during co-culture with macrophages. Exp Cell Res. 1985 Sep;160(1):150–157. doi: 10.1016/0014-4827(85)90244-7. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Cleavage of macromolecular heparin by an enzyme from mouse mastocytoma. J Biol Chem. 1975 Apr 10;250(7):2690–2697. [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Degradation of heparin in mouse mastocytoma tissue. Biochem J. 1971 Dec;125(4):1119–1129. doi: 10.1042/bj1251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Metabolism of macromolecular heparin in mouse neoplastic mast cells. Biochem J. 1976 Mar 15;154(3):605–611. doi: 10.1042/bj1540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Heldin C. H., Wasteson A., Busch C., Hök M. Characterization of a platelet endoglycosidase degrading heparin-like polysaccharides. Biochemistry. 1980 Dec 9;19(25):5755–5762. doi: 10.1021/bi00566a014. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Wasteson A., Robinson H. C., Ogren S., Lindahl U. Enzymatic depolymerization of heparin-related polysaccharides. Substrate specificities of mouse mastocytoma and human platelet endo-beta-D-glucuronidases. J Biol Chem. 1982 Sep 10;257(17):10278–10282. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Metabolism of proteoglycans in rat ovarian granulosa cell culture. Multiple intracellular degradative pathways and the effect of chloroquine. J Biol Chem. 1984 Aug 25;259(16):10270–10283. [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]


