Abstract
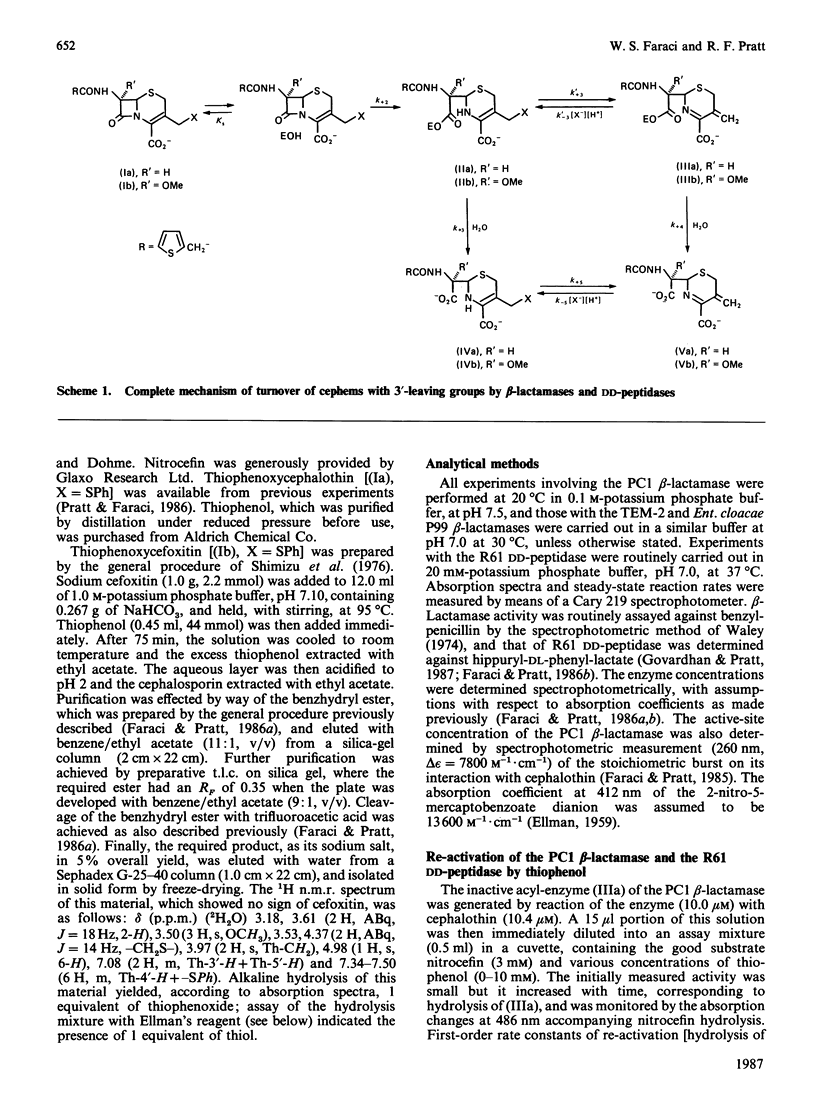
It has been shown previously [Faraci & Pratt (1985) Biochemistry 24, 903-910; (1986) Biochemistry 25, 2934-2941; (1986) Biochem. J. 238, 309-312] that certain beta-lactam-processing enzymes form inert acyl-enzymes with cephems that possess good leaving groups at the C-3' position. These inert species arise by elimination of the leaving group from the initially formed and more rapidly hydrolysing acyl-enzyme, which has the 'normal' cephalosporoate structure. The present paper shows that a strong nucleophile, thiophenoxide, can catalyse the re-activation of three examples of these inert acyl-enzymes, generated on reaction of cephalothin and cefoxitin with the PC1 beta-lactamase of Staphylococcus aureus and of cephalothin with D-alanyl-D-alanine transpeptidase/carboxypeptidase of Streptomyces R61. In view of the reversibility of the elimination reaction, demonstrated in model systems [Pratt & Faraci (1986) J. Am. Chem. Soc. 108, 5328-5333], this catalysis is proposed to arise through nucleophilic addition to the exo-methylene carbon atom of the inert acyl-enzyme to regenerate a more rapidly hydrolysing normal cephalosporoate. Strong support for this scenario was obtained through comparison of the kinetics of the catalysed re-activation reaction with those of turnover of the relevant 3'-thiophenoxycephems, thiophenoxycephalothin and thiophenoxycefoxitin. The enzymes appear to stabilize the products of the elimination reaction with respect to the normal cephalosporoate, but more strongly to destabilize the transition states. The effects of other nucleophiles, including cysteine, glycine amide and imidazole, on the above enzymes and on other beta-lactamases can be understood in terms of the model reaction kinetics and thermodynamics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. Observation of a covalent intermediate during turnover of a fluorescent cephalosporin by the beta-lactamase of STaphylococcus aureus PC1. J Biol Chem. 1981 Nov 25;256(22):11401–11404. [PubMed] [Google Scholar]
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. The trapping of a covalent intermediate and the interpretation of pH rate profiles. J Biol Chem. 1983 Nov 10;258(21):13120–13126. [PubMed] [Google Scholar]
- Boyd D. B., Lunn W. H. Electronic structures of cephalosporins and penicillins. 9. Departure of a leaving group in cephalosporins. J Med Chem. 1979 Jul;22(7):778–784. doi: 10.1021/jm00193a006. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Interactions of cephalosporins with the Streptomyces R61 DD-transpeptidase/carboxypeptidase. Influence of the 3'-substituent. Biochem J. 1986 Aug 15;238(1):309–312. doi: 10.1042/bj2380309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Mechanism of inhibition of RTEM-2 beta-lactamase by cephamycins: relative importance of the 7 alpha-methoxy group and the 3' leaving group. Biochemistry. 1986 May 20;25(10):2934–2941. doi: 10.1021/bi00358a030. [DOI] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Mechanism of inhibition of the PC1 beta-lactamase of Staphylococcus aureus by cephalosporins: importance of the 3'-leaving group. Biochemistry. 1985 Feb 12;24(4):903–910. doi: 10.1021/bi00325a014. [DOI] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Govardhan C. P., Pratt R. F. Kinetics and mechanism of the serine beta-lactamase catalyzed hydrolysis of depsipeptides. Biochemistry. 1987 Jun 16;26(12):3385–3395. doi: 10.1021/bi00386a021. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Dideberg O., Charlier P., Wery J. P., Libert M., Moews P. C., Knox J. R., Duez C., Fraipont C., Joris B. On the origin of bacterial resistance to penicillin: comparison of a beta-lactamase and a penicillin target. Science. 1986 Mar 21;231(4744):1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Govardhan C. P. beta-Lactamase-catalyzed hydrolysis of acyclic depsipeptides and acyl transfer to specific amino acid acceptors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1302–1306. doi: 10.1073/pnas.81.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu B., Kaneko M., Kimura M., Sugawara S. Synthesis of 7 alpha-methoxy-7-[2-(substituted thio)acetamido]cephalosporin derivatives and their antibacterial activities. Chem Pharm Bull (Tokyo) 1976 Nov;24(11):2629–2636. doi: 10.1248/cpb.24.2629. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]


