Abstract
The glucose-transport protein from bovine cerebral-cortex microvessels has been identified and characterized by virtue of its ability to bind the ligand [4-3H]cytochalasin B. Microvessel membranes were found to contain a single set of glucose-inhibitable high-affinity cytochalasin B-binding sites [113 +/- 16 (S.E.M.) pmol/mg of membrane protein], with an association constant of 6.8 +/- 1.8 (S.E.M.) micron-1. D-Glucose inhibited the binding to these sites with a Ki of 31 mM. The transport protein was identified by photoaffinity labelling with [4-3H]cytochalasin B and was found to migrate as a broad band of apparent Mr 55,000 on SDS/polyacrylamide gels. Labelling was inhibited by D-glucose, but not by L-glucose. Treatment with endoglycosidase F yielded a sharper band of apparent Mr 46,000, indicating that the transport protein is glycosylated. However, in contrast with the human erythrocyte glucose transporter, digestion with endo-beta-galactosidase had little effect on the electrophoretic mobility of the microvessel protein. Tryptic digestion of the photolabelled protein yielded a radioactive fragment of apparent Mr 18,000, similar to that of the fragment produced by digestion of the labelled human erythrocyte glucose transporter. In addition, a protein of Mr identical with that of the photolabelled transporter was labelled on Western blots of microvessel membranes by antisera raised against the intact erythrocyte transporter and against synthetic peptides corresponding to its N- and C-terminal regions. It is concluded that the glucose-transport protein of bovine cerebral-cortex microvessel endothelial cells shows structural homology with the human erythrocyte glucose transporter.
Full text
PDF



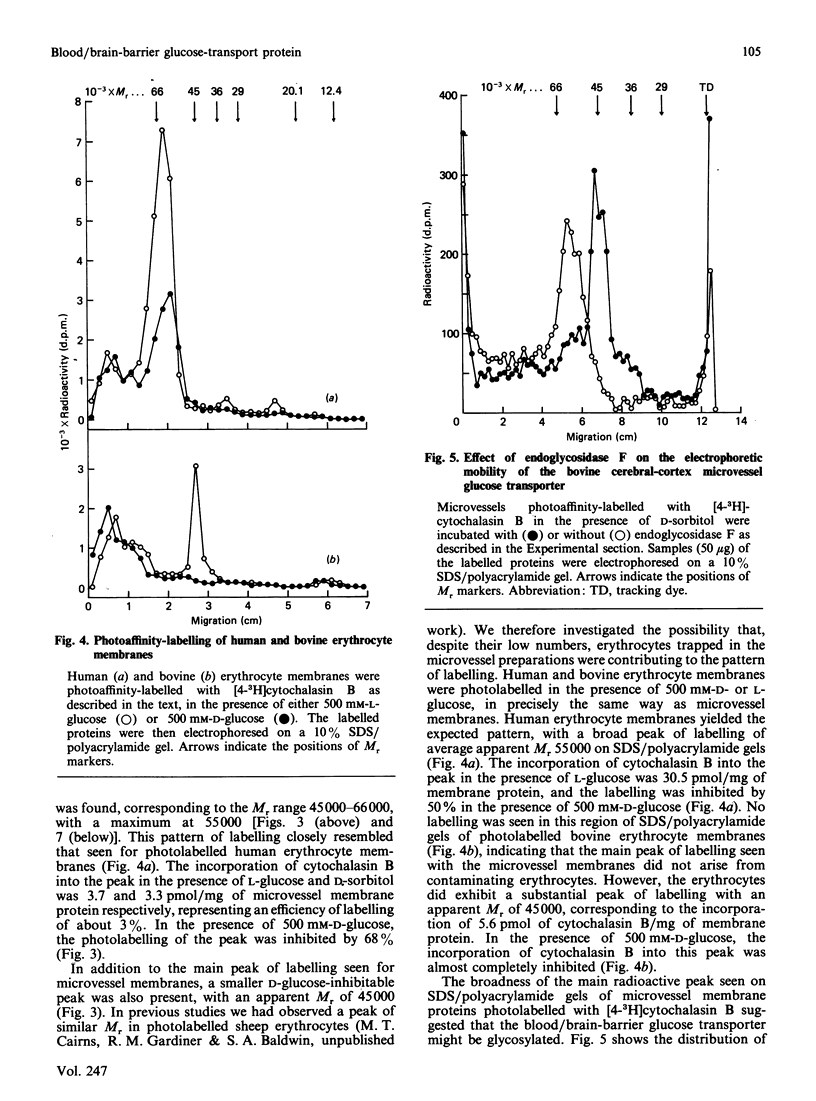
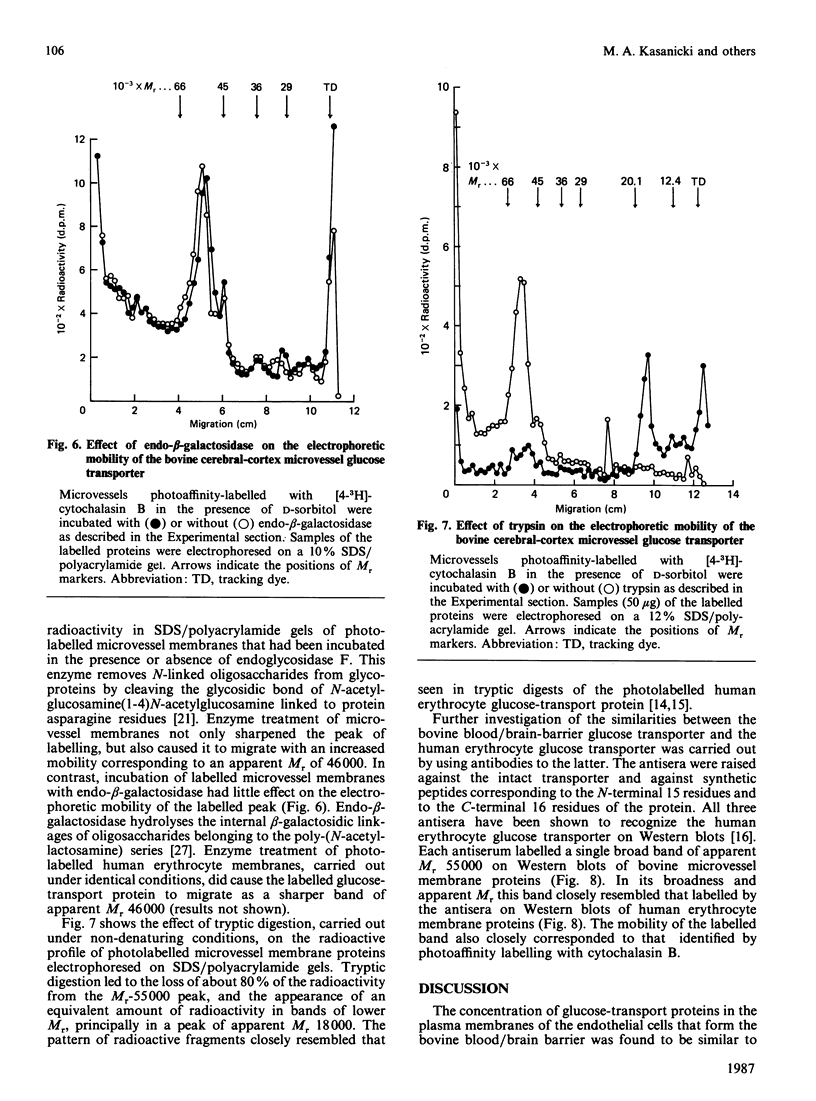
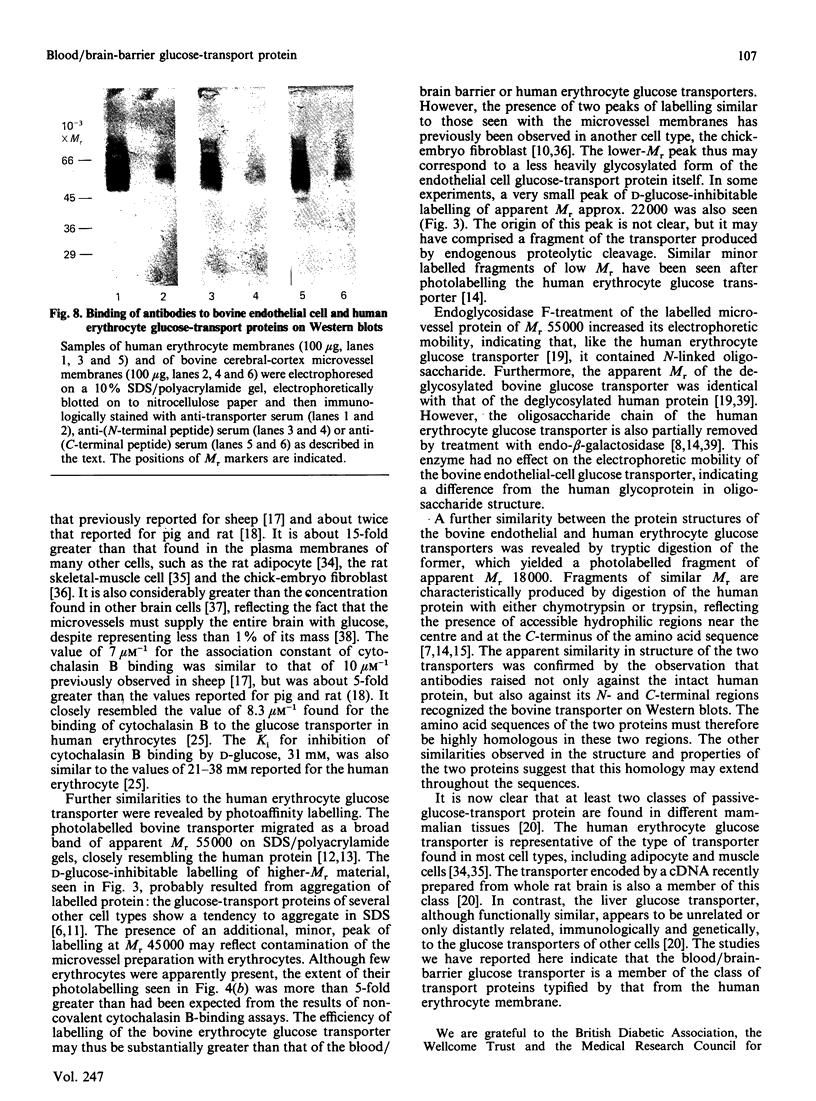

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert Z., Orlowski M., Rzucidlo Z., Orlowska J. Studies on gamma-glutamyl transpeptidase activity and its histochemical localization in the central nervous system of man and different animal species. Acta Histochem. 1966;25(5):312–320. [PubMed] [Google Scholar]
- Allard W. J., Lienhard G. E. Monoclonal antibodies to the glucose transporter from human erythrocytes. Identification of the transporter as a Mr = 55,000 protein. J Biol Chem. 1985 Jul 25;260(15):8668–8675. [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Cairns M. T., Gardiner R. M., Ruggier R. A D-glucose-sensitive cytochalasin B binding component of cerebral microvessels. J Neurochem. 1985 Aug;45(2):650–652. doi: 10.1111/j.1471-4159.1985.tb04039.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Whetton A. D., Dexter T. M., Meeran K., Baldwin S. A. Characterisation of monoclonal antibodies which specifically recognise the human erythrocyte glucose transport protein. EMBO J. 1985 Dec 1;4(12):3093–3098. doi: 10.1002/j.1460-2075.1985.tb04050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Davies A., Meeran K., Cairns M. T., Baldwin S. A. Peptide-specific antibodies as probes of the orientation of the glucose transporter in the human erythrocyte membrane. J Biol Chem. 1987 Jul 5;262(19):9347–9352. [PubMed] [Google Scholar]
- Deziel M. R., Rothstein A. Proteolytic cleavages of cytochalasin B binding components of band 4.5 proteins of the human red blood cell membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):10–20. doi: 10.1016/0005-2736(84)90245-1. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I. Distribution of the glucose transporter in the mammalian brain. J Neurochem. 1986 May;46(5):1406–1411. doi: 10.1111/j.1471-4159.1986.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I., Klip A., Walker D. M. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D., Matzura H. An improved method of counting radioactive acrylamide gels. Anal Biochem. 1971 Aug;42(2):481–486. doi: 10.1016/0003-2697(71)90062-5. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D. The glucose transport system of muscle plasma membranes: characterization by means of [3H]cytochalasin B binding. Arch Biochem Biophys. 1983 Feb 15;221(1):175–187. doi: 10.1016/0003-9861(83)90134-0. [DOI] [PubMed] [Google Scholar]
- Kwong F. Y., Baldwin S. A., Scudder P. R., Jarvis S. M., Choy M. Y., Young J. D. Erythrocyte nucleoside and sugar transport. Endo-beta-galactosidase and endoglycosidase-F digestion of partially purified human and pig transporter proteins. Biochem J. 1986 Dec 1;240(2):349–356. doi: 10.1042/bj2400349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Pessin J. E., Tillotson L. G., Yamada K., Gitomer W., Carter-Su C., Mora R., Isselbacher K. J., Czech M. P. Identification of the stereospecific hexose transporter from starved and fed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2286–2290. doi: 10.1073/pnas.79.7.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P., Uemura K., Dolby J., Fukuda M. N., Feizi T. Isolation and characterization of an endo-beta-galactosidase from Bacteroides fragilis. Biochem J. 1983 Aug 1;213(2):485–494. doi: 10.1042/bj2130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., Olson S. A., Weber M. J., Lienhard G. E., Gorga J. C. Photolabeling of glucose-sensitive cytochalasin B binding proteins in erythrocyte, fibroblast and adipocyte membranes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):38–43. doi: 10.1016/0006-291x(82)91666-7. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Wagner R., Zimmer G., Lacko L. An interspecies approach to the investigation of the red cell membrane glucose transporter. Biochim Biophys Acta. 1984 Mar 28;771(1):99–102. doi: 10.1016/0005-2736(84)90115-9. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M., Robins M. J., Paterson A. R. Photoaffinity labeling of the human erythrocyte nucleoside transporter by N6-(p-Azidobenzyl)adenosine and nitrobenzylthioinosine. Evidence that the transporter is a band 4.5 polypeptide. J Biol Chem. 1983 Feb 25;258(4):2202–2208. [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]