Abstract
Background and Objective
Pancreatic cancer (PC) is a significant cause of cancer-related mortality, with limited curative options and high rates of cachexia, a debilitating syndrome associated with poor prognosis. While previous research has linked sarcopenia to poor outcomes in PC, the correlation between cachexia and treatment outcomes remains underexplored. This meta-analysis aims to investigate the association between cachexia and overall survival and time to treatment failure in advanced PC patients undergoing first-line chemotherapy.
Method
A systematic search of electronic databases was conducted following PRISMA guidelines. Eligible studies compared cachexic and non-cachexic PC patients, reporting outcomes of observed survival or time to treatment failure. Data extraction and analysis were performed using Comprehensive Meta-Analysis Version 3.3, employing random-effects models and sensitivity analyses to assess heterogeneity and bias.
Results
Seven observational studies involving 2834 PC patients were included. The incidence of cachexia was 45% (95% CI: 0.27-0.65), with a higher prevalence in East Asian populations. Cachexic patients experienced significantly earlier treatment failure (SDM: −2.22, 95% CI: −2.6 to −1.7, P = 0.0001) and higher mortality risk (HR: 2.02, 95% CI: 1.17-3.48, P = 0.011) compared to non-cachexic patients. Overall survival was lower in cachexic patients (SDM: −2.34, 95% CI: −3.7 to −0.90, P = 0.001), with considerable heterogeneity across studies. Meta-regression analysis revealed significant differences between countries but insignificant correlations with age.
Conclusion
Cachexia is associated with reduced overall survival, early chemotherapy failure, and elevated mortality in advanced PC patients undergoing first-line chemotherapy. Recognition and management of cachexia are crucial for optimizing treatment outcomes and improving patient survival. Future research should focus on prospective studies to better understand the impact of cachexia on treatment response and develop tailored interventions to mitigate its adverse effects.
Keywords: pancreatic cancer, cachexia, chemotherapy, overall survival, treatment failure, meta-analysis, prognosis, observational studies, mortality, East Asian populations
Plain language summary
Pancreatic cancer (PC) presents a formidable challenge due to its limited treatment options and association with cachexia, a debilitating condition linked to poor prognosis. This meta-analysis investigates the relationship between cachexia and treatment outcomes in advanced PC patients undergoing first-line chemotherapy. Seven observational studies encompassing 2834 patients were analyzed, revealing a 45% incidence of cachexia, notably higher in East Asian populations. Cachexic patients exhibited earlier treatment failure and higher mortality risk compared to non-cachexic counterparts. Their overall survival was significantly reduced, although with notable heterogeneity across studies. Meta-regression analysis highlighted variations between countries but found no significant correlation with age. The findings underscore the importance of recognizing and addressing cachexia to optimize treatment outcomes and enhance patient survival. Future research should emphasize prospective studies to further elucidate cachexia’s impact on treatment response and develop tailored interventions to alleviate its adverse effects.
Introduction
Pancreatic cancer (PC) is the third leading cause of cancer-related deaths in the United States, with projections that it will become the second leading cause by the year 2030. 1 Surgical resection is the only curative option spite–80%–85% of individuals presenting with advanced stages.2-4 The combination regimen of FOLFIRINOX (5-fuorouracil, leucovorin, irinotecan, and oxaliplatin5,6 and gemcitabine with nab-paclitaxel (GnP) are the usual first-line therapies for advanced PC patients with good performance status.7,8 Extensive combination regimens improve the rate of survival but also cause adverse effects and require proper patient selection.9,10
Cancer Cachexia is a multifactor syndrome comprised of weight loss, diminished physical well-being, low quality of life, and intolerance to cancer treatment.11-13 It is reported in 50%-80% of patient with various cancers, with the highest prevalence in pancreatic cancer (60%). 13 The diagnostic criteria for cachexia include either a weight loss of ≥5% within 6 months, a weight loss greater than 2% accompanied by a BMI of <20 kg/m2, or the presence of sarcopenia.14,15 Sarcopenia is characterised by progressive loss of muscle mass and function, along with loss of the entire body mass.16,17 The prevalence of sarcopenia in PC patients is up to 50%, while in other common cancers, it is about 40%. 18 Cachexia and Sarcopenia are 2 different entities with overlapping characteristics of muscular deficiency illnesses that induce disability, functional impairment, and decreased physical performance. 19 Moreover, cachexia and sarcopenia reduce tolerance to chemotherapy and increase the risk of adverse effects in patients with advanced pancreatic cancer.20,21 Both cachexia and sarcopenia are correlated with poor prognosis in individuals with malignancies, particularly PC. However, this correlation varies according to cancer type and stage. 22
There is currently a scarcity of research that assesses the part that cachexia plays among patients with PC, and it is worth noting that recent studies have demonstrated that cachexia is highly prevalent in individuals who have advanced PC23,24 it is important to understand the correlation in pancreatic patients. While a systematic review and meta-analysis has already been conducted on effect of sarcopenia on treatment of pancreatic cancer, 25 cancer cachexia owning to the high prevalence in pancreatic cancer warrants a thorough data analysis to know the extent of impact it contributes to treatment outcomes, ultimately starting a debate for future management and reaching a better 5 years mortality rate. The aim of our study was to determine the association between cachexia and overall survival and time to treatment failure. By examining these associations, we enhanced our understanding of how cachexia influences the prognosis and treatment outcomes of PC, potentially leading to improved clinical management strategies.
Methodology
Search Strategy
A comprehensive search was carried out by S.M.S.A and A.J across electronic databases, including PubMed, MEDLINE, Embase, and Scopus, from the inception date until 1 April 2024. The search was conducted using the MeSH phrases “Cachexia”, “Pancreatic cancer”, “Body Weight loss”, “Advanced stage cancer”, and “Palliative chemotherapy”, with English as the only language used for the data search. Additional sources were consulted to identify relevant records. The search was conducted in English, adhering to the spelling and specific terms and phrases. The guidelines for systematic reviews and meta-analyses, specifically the MOOSE, 26 were followed while also incorporating the PRISMA principles. 27 The protocol for this study has been registered in PROSPERO (CRD42024545153).
Eligibility Criteria
The following criteria were considered when incorporating relevant studies: (i) Adult patients with locally advanced, metastatic , unresectable or resectable pancreatic cancer who were either intolerant to or refused surgery and were starting first-line palliative chemotherapy.; (ii) the study must have compared cancer cachexia ie, A multifactorial syndrome involving a weight loss of 5% or more within 6 months, OR 2% weight loss in individuals with low BMI and leads to irreversible fat mass loss , skeletal muscle loss and functional impairment, with non-cachexia patients; and (iii) studies were only included if outcomes of observed survival (OS) or time to treatment failure (TTF) were reported.
Studies that included cancer types other than pancreatic cancer, used sarcopenia cohorts instead of cachexia, or did not compare non-cachexia patients as a cohort were excluded.
Outcome Measures and Data Extraction
Two authors, M.A.S and A.J, independently gathered key information from the selected studies. After extraction, the data were reviewed in duplicate. The outcomes of interest were primarily time to treatment failure and observed survival. Secondary outcomes included cachexia as a risk factor for mortality and incidence of cachexia and sarcopenia in the included studies, and months were converted to days for consistency.
Statistical Analysis
The Comprehensive Meta-Analysis Version 3.3 was employed by M.A.S to combine and analyze the data. To calculate the pooled effects between the control and intervention groups, a random-effects model was used. Forest plots displaying the outcomes of time to treatment failure and overall survival were created using the standardized difference in means and 95% confidence intervals (CIs). Furthermore, separate scatter plots were generated to examine the regression of age and country against the standardized difference in mean for overall survival. Forest plots illustrating the incidence rates of cachexia and sarcopenia were constructed using event rates and 95% CIs. Hazard ratios and 95% confidence intervals (CIs) were used to analyze the risk of mortality due to cachexia. Heterogeneity was assessed using I2 statistics, and significance was considered when I2 exceeded 50%. Additionally, the leave-one-out methodology was employed when necessary to identify specific studies that contributed to the observed variation, thereby enhancing the accuracy and reliability of the research results.
Quality Assessment and Risk of Bias
A.R and S.M.S.A evaluated the quality of the observational studies using the Newcastle-Ottawa quality assessment scale (Supplemental Figure 1). 28 Biases in selection, comparability, and outcomes were also considered. For each study, a total score of less than 7 out of 9 was considered to indicate a low bias. Any difficulties that arose during the process were resolved by a third researcher, M.A.S
Results
Study Selection
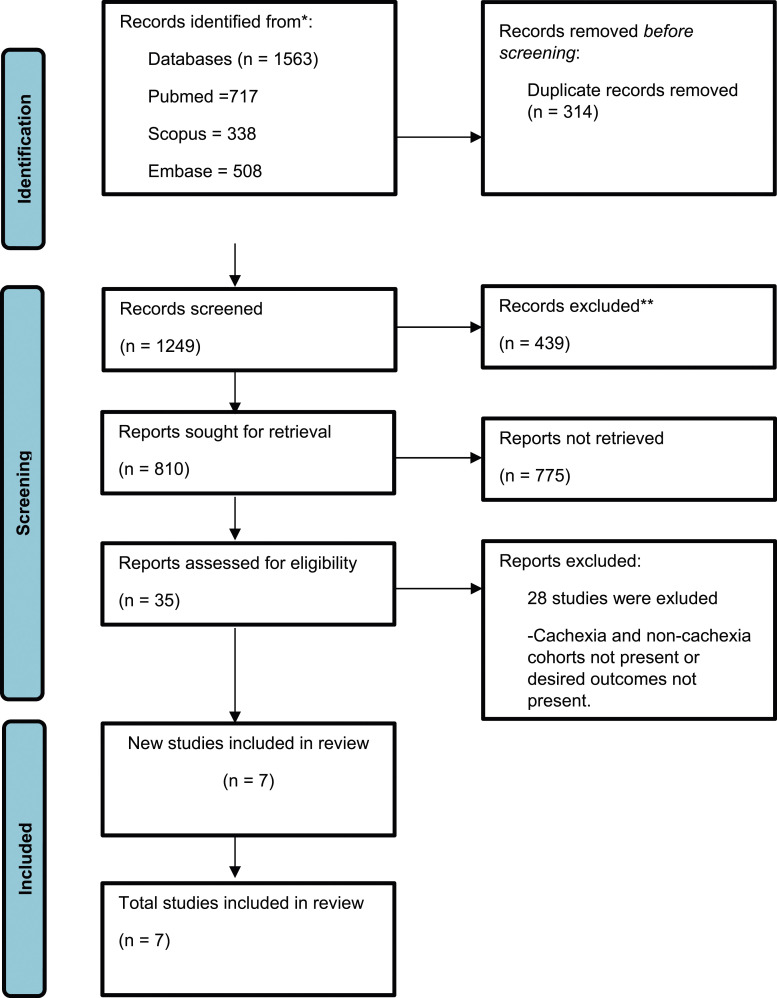
The PRISMA flow diagram clearly illustrates the search strategy outcomes (Figure 1). Initially, 1563 articles were retrieved from multiple databases. Subsequently, 314 duplicate articles were excluded. The remaining 1249 articles underwent preliminary screening, of which 439 were excluded based on their titles alone. Furthermore, the remaining 810 studies were assessed, and 775 were excluded based on the abstracts, allowing us to remove studies that were not aligned with our PICO. At the end of screening, 35 studies were assessed for eligibility. Among these, 28 studies were omitted because of the inclusion of patients who underwent surgery for pancreatic cancer alongside other cancer types, such as colorectal cancer, lung cancer, or gastric cancer. Additionally, a few studies failed to provide results for time-to-treatment failure (TTF) or overall survival (OS), whereas certain studies specifically targeted patients with sarcopenia. Eventually, 7 observational studies were deemed appropriate after a meticulous screening process that closely matched the aims of our research23,24,29-33 (Tables 1 and 2).
Figure 1.
PRISMA flowchart demonstrating search strategy of present systemic review and meta-analysis.
Table 1.
Baseline Characteristics of Included Studies.
| Author | Country | Time Window (Years) | Sample Size | Age (Mean (SD)) | Gender | Inclusion Criteria | Definition of Cachexia | Tumor Location | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cachexia | Non-cachexia | Cachexia | Non-cachexia | Cachexia | Non-cachexia | Cachexia | Non-Cachexia | |||||
| Junji useFur 2024 | Japan | 2 | 421 | 1476 | 68.7 ± 8.9 | 67.7 ± 9.2 | Male 249 female 172 | Male 835 female 641 | Definitive diagnosis of invasive ductal carcinoma of the pancreas. FFX or GnP regimen as frst-line chemotherapy started at age ≥20 years | Diagnostic criteria for cachexia is ≥5% weight loss in 6 months | NR | NR |
| Tsuyoshi Takeda 2021 | Japan | 5.2 | 48 | 32 | 79.5 ± 3.6 | 78.5 ± 3.8 | Male 21 female 27 | Male 14 female 18 | Consecutive patients ≥75 years of age with pathologically confirmed metastatic PC who received chemotherapy | Cachexia is a multifactorial syndrome characterized by weight loss with concomitant loss of muscle and/or fat mass | Ph:44% Pbt:56% | Ph:44% Pbt:56% |
| Henry C.Y 2019 | Hong Kong | 6 | 14 | 58 | 67.86 ± 5.6 | 64.12 ± 6.4 | Male 9 Female 5 | Male 36 Female 22 | Diagnosed pancreatic cancer preferably by histologically and who were scheduled to undergo chemotherapy of palliative intent and Chinese ethnicity. | Weight loss of 5% or higher from basal body weight, which was frequently termed critical weight loss (CWL) | Ph:42% Pbt:57.1% | Ph:60.3% Pbt:39.7% |
| Kana Hosokawa, M 2023 | Japan | 9 | 95 | 125 | 73.66 ± 7.40 | 75.33 ± 10.37 | Male 48 Female 47 | Male 60 Female 65 | Diagnosed and treated for metastatic, locally advanced, or resectable PC who were intolerant to or refused surgery | Cancer cachexia is diagnosed when weight loss is greater than 5% or weight loss is greater than 2% in individuals already showing depletion as assessed by current BMI | Ph:40.1% Pbt:56.8% | |
| Ya-Chin Hou 2022 | Taiwan | 10 | 145 | 27 | 65.21 ± 9.2 | 63.37 ± 3.6 | Male 124 Female 21 | Male 25 Female 2 | Advanced PC patients were enrolled in this study | Weight loss greater than 5% over past 6 months or weight loss greater than 2% in individuals already showing depletion according to current BMI | Ph:40.2% Pbt:59.8% | Ph:39.5% Pbt:60.5% |
| Yukari S uzuki 2023 | Tokyo | 5.6 | 84 | 54 | 68.3 ± 10 | 65.73 ± 9.78 | Male 43 Female 41 | Male 37 Female 17 | Patients with unresectable histologically or cytologically diagnosed as pancreatic ductal adenocarcinoma who started GnP as frst-line chemotherapy | Cancer cachexia is defined by loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment (SMI change rate ≥−3.5%) | NR | NR |
| Lindsay Carnie 2020 | UK | 4 | 59 | 196 | 62.66 ± 31.85 | 59 ± 44.4 | Male 39 Female 20 | Male 100 Female 96 | Newly-diagnosed patients with advanced PC starting first-line palliative chemotherapy | Weight loss of >5% in 4weeks | Ph:62.7% Pbt:37.3% | Ph:58.2% Pbt:41.8% |
Ph: pancreatic head; Pbt: pancreatic body and tail; GnP: gemcitabine plus nab-paclitaxel; FFX: FOLFIRINOX.
Table 2.
Overall Results Summary.
| No. of studies | Variable | Pooled Result | 95% CI | P value | Heterogeneity (%) | ||
|---|---|---|---|---|---|---|---|
| Incidence of prevalence of cachexia | 7 | ER | 0.45 | 0.27-0.65 | P = 0.657 | I2 = 98.13 | P = 0.001 |
| Incidence of prevalence of sarcopenia | 3 | ER | 0.57 | 0.40-0.72 | P = 0.418 | I2 = 91.2 | P = 0.001 |
| Time to treatment failure | 5 | SMD | −2.22 | −2.6 to −1.7 | P = 0.000 | I2 = 78.15 | P = 0.003 |
| Cachexia as a risk factor for mortality | 3 | HR | 2.02 | 1.17-3.48 | P = 0.011 | I2 = 45.39 | P = 0.160 |
| Overall survival | 5 | SMD | −2.34 | −3.7 to −0.90 | P = 0.001 | I2 = 98.08 | P = 0.00 |
Study Characteristics
Our research encompassed a selection of 7 studies, which collectively involved 2834 pancreatic cancer patients, of which 866 had cachexia and 1968 were non-cachexic. The mean age of cachexic patients was 69.3 years, whereas that of non-cachexic patients was 67.6 years. The cachexic group comprised 533 males and 333 females, while the non-cachexic group comprised 1107 males and 861 females. The majority of these studies (85.7%) were conducted in East Asian countries, with only 1 study (14.2%) originating from Northwestern Europe. Within this subset, 5 studies were retrospective in design. In 2 of these studies by Wong et al. 23 and Hosokawa et al., 24 2 specific medications, referred to as FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) (FFX) and gemcitabine and nab-paclitaxel (GnP) were utilized. Notably, in the study by Wong et al, 23 detailed median doses of 5 and 9 for cachexic patients and 7 and 11 for non-cachexic patients were administered. In the latter group, both cachexic and non-cachexic patients received 4 doses each of FFX and GnPs.
Incidence
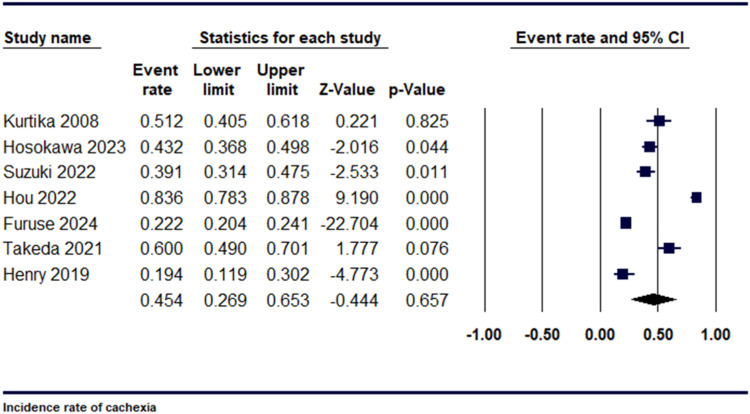
In our research, we examined 7 studies to assess the prevalence of cachexia in individuals with pancreatic cancer. The incidence rate was 0.45 [95% CI: 0.27-0.65, P = 0.657], as shown in Figure 2. Upon conducting a sensitivity analysis, significant heterogeneity was observed among the studies, for which a leave-one- out analysis was performed. These studies included Hou et al and Henry et al, (I2 = 98.13%, P = 0.001) (Supplemental Figure 2).
Figure 2.
Forest plot demonstrating Incidence rate of cachexia.
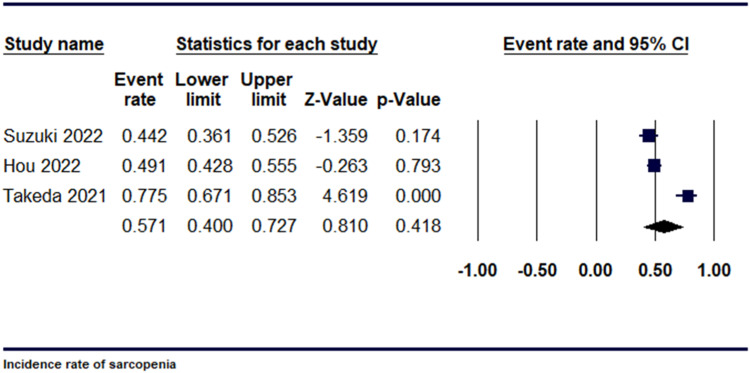
Additionally, we analysed 3 studies to determine the prevalence of sarcopenia in individuals with pancreatic cancer. The event rate was 0.571 [95% CI, 0.40-0.72, P = 0.418], as shown in Figure 3. Upon conducting a sensitivity analysis, using leave 1 out analysis, substantial heterogeneity was present among the studies, (I2 = 91.20%, P = 0.001). (Supplemental Figure 3).
Figure 3.
Forest plot demonstrating Incidence rate of sarcopenia.
Time to Treatment Failure
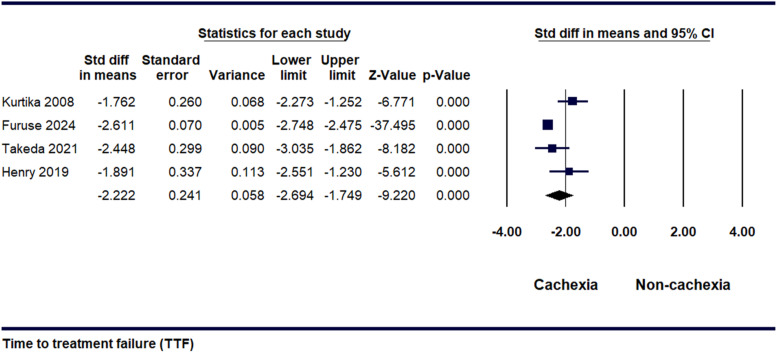
Five studies provided data on determining the time to TTF in cachexic and non-cachexic patients. The SDM was discovered to be −2.22 [95% CI, −2.6 to-1.7, P = 0.000], as illustrated in Figure 4. It was inferred from the results that cachexic patients experienced early treatment failure. Following a sensitivity analysis, it was observed that significant heterogeneity existed among the studies [I2 = 78.15%, P = 0.003] (Supplemental Figure 4).
Figure 4.
Forest plot illustrating the standardized difference in means for time to treatment failure between patients with cachexia and non-cachexia.
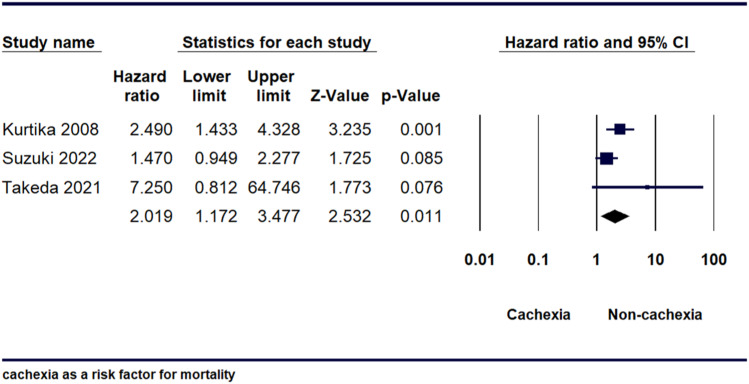
Cachexia as a Risk Factor for Mortality
Three studies showed that cachexia is a risk factor for mortality. The H.R calculated was 2.02 [95% CI, 1.17-3.48, P = 0.011], indicating that mortality risk was substantially higher in the cachexia group compared to the non-cachexia group (Figure 5). After conducting a sensitivity analysis, it was observed that there was no significant heterogeneity among the studies [I2 = 45.39%, P = 0.160] as shown in (Supplemental Figure 5).
Figure 5.
Forest plot illustrating mortality hazard ratio between Cachexia and Non cachexia.
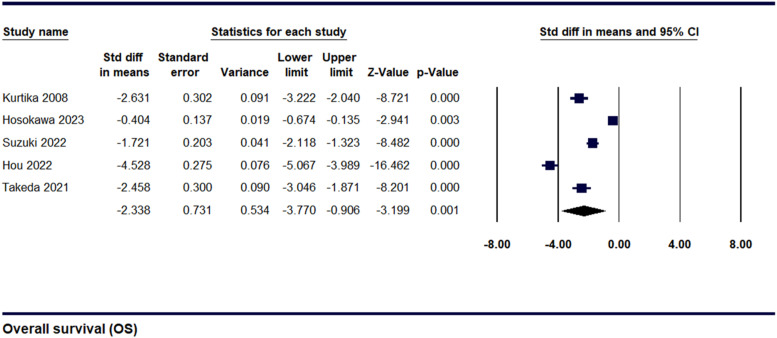
Overall Survival (OS) for Cachexia
Data were gathered from 5 studies to evaluate the overall survival among cachexic and non-cachexic pancreatic cancer patients. The SDM was found to be −2.34 ± 0.73 [95% CI, −3.7 to −0.90, P = 0.001], as shown in Figure 6 indicating that cachexic patients had lower OS than non-cachexic patients. Sensitivity analysis demonstrated that significant heterogeneity was present [I2 = 98.08%, P = 0.00], as shown in (Supplemental Figure 6). To investigate the cause of this significant heterogeneity, the leave-one-out method was employed, after which the values remained almost similar at-2.34 ± 0.73 [95% CI, −3.7 to −0.90, P = 0.001]. (Supplemental Figure 7)
Figure 6.
Forest plot illustrating Overall survival for Cachexia and Non-cachexia.
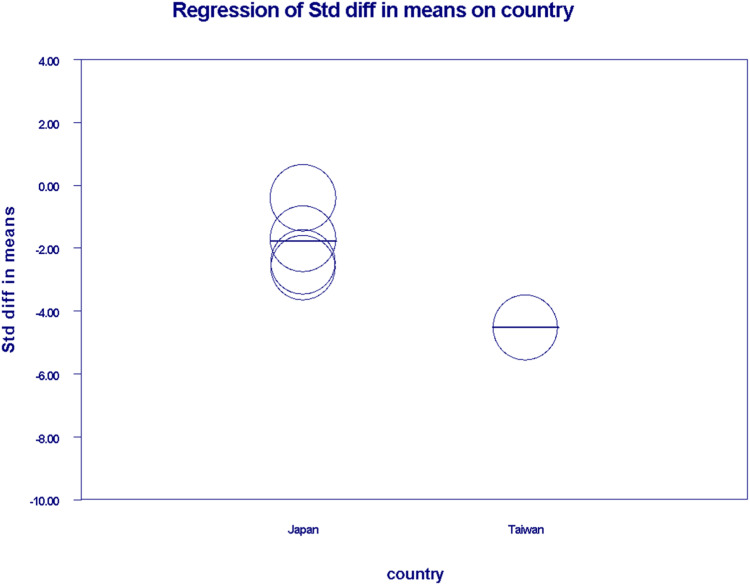
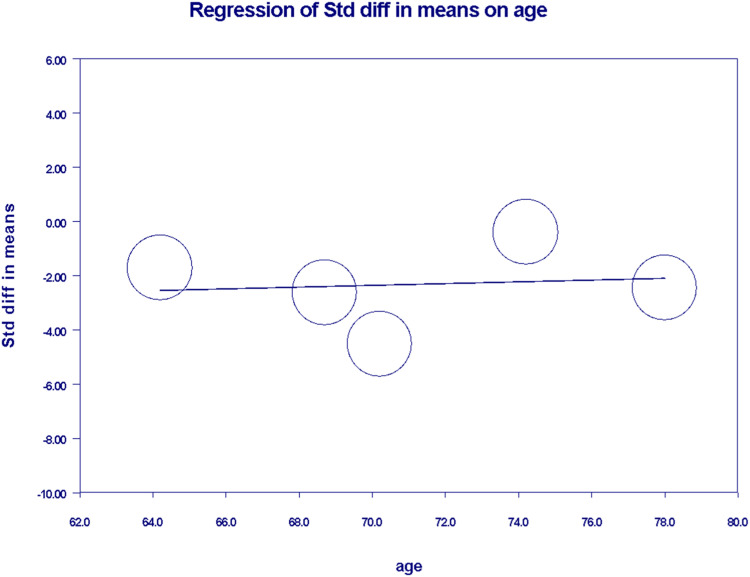
In the meta-regression analysis, we generated separate scatter plots to investigate the relationship between OS and country as well as age. Japan exhibited mixed and higher coefficients, indicating its prominence as an inception country for a greater number of studies. Conversely, the coefficient for Taiwan was determined to be −2.74 ± 0.24 [95% CI (−5.24 to −0.24)] with a two-sided (P = 0.0315) suggesting significant correlation (Figure 7). Upon performing sensitivity analysis, significant heterogeneity was found [I2 = 96.2%, P = 0.00] as shown in (Supplemental Figure 8). Regarding ages 64-79, the coefficient was calculated to be 0.03 ± 0.18 [95% CI, −0.32 to 0.38, P = 0.856] as shown in (Figure 8), suggesting an insignificant correlation between age and overall survival. On conducting sensitivity analysis, significant heterogeneity was present among studies [I2 = 98.41%, P = 0.00] (Supplemental Figure 9).
Figure 7.
Scatter plot demostrating Meta-Regression for overall survival against country of origin.
Figure 8.
Scatter plot demonstrating Meta-regression for overall survival according to age.
Discussion
The primary aim of this meta-analysis was to provide a comprehensive overview of the impact of cancer cachexia on first-line chemotherapy for patients with advanced pancreatic cancer. This is particularly significant given the high mortality rates associated with the disease, its detrimental effect on quality of life, and the added burden on the health care system resulting from treatment delays in cachexia patients when compared to non-cachexia patients. Our study’s objective is to summarize existing evidence in order to offer critical insights that can inform clinical practice and support the development of targeted interventions for this vulnerable patient population.
Overall, we found evidence from 7 studies that cachexia significantly correlates with reduced overall survival, increased likelihood of early chemotherapy failure, and elevated mortality rates compared to patients without cachexia. Significant heterogeneity was present among studies for the incidence of cachexia, TTF, risk of mortality, and OS particularly.
A study described PC cachexia as a ‘three-dimensional syndrome’ comprising the impact of tumours-related factors on the body, the role of the pancreas in digestion (exocrine and endocrine insufficiency), and the anatomical and physiological association between the pancreas and other organs in the gastrointestinal tract. 14 The clinical course of patients undergoing chemotherapy is less affected by sarcopenia than by cachexia. Some of the studies in our analysis highlighted nutritional parameters such as total protein and albumin and inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR), serum C-reactive protein (CRP), and platelet-to-lymphocyte ratio (PLR) as potential predictors of cachexia in patients receiving chemotherapy.30,32 Furthermore, systemic inflammation associated with cancer plays a pivotal role in the onset and advancement of cachexia, whereas sarcopenia is characterised by a lack of inflammation and potential reversibility. 13 While our analysis showed no significant impact of sarcopenia, unlike cachexia, another study investigated the influence of sarcopenia on both OS and chemotherapy toxicity in pancreatic cancer patients undergoing chemotherapy, revealing a notable correlation. 14
Overall, a significant correlation was observed between cachexia and early chemotherapy failure. However, our analysis also revealed considerable heterogeneity in early treatment failure across the studies included. In particular, in an investigation by Furuse, 33 the FFX regimen demonstrated a reduced TTF in cachexia patients compared to non-cachexia patients, whereas no such dissimilarity was observed among patients receiving the GnP regimen. This difference may be attributed to the higher incidence of side effects such as severe anorexia and diarrhea associated with FFX compared to GnP. Patients with cachexia, weakened by their condition and associated side effects, have a reduced tolerance to chemotherapy.34,35 Psychological distress induced by depression and anxiety further influences treatment compliance and responses.36,37
Cachexia can affect drug metabolism and pharmacokinetics, leading to either reduced drug efficacy or increased toxicity.38,39 Growing evidence explains how certain chemotherapeutic agents may play a role in the advancement of cachexia. 40 Once chemotherapy-induced toxicity exacerbates cachexia, chemotherapy failure ensues, creating a vicious cycle. Therefore, ensuring the proper dosing of chemotherapeutic agents is essential for treatment success.
A study by Hosokawa 24 found no association between weight loss at diagnosis and prognosis in patients with advanced pancreatic cancer treated with best supportive care or chemotherapy. This is attributed to the finding that patients who did not lose weight had better survival rates in the middle of the observation period, regardless of whether they received basic regular care or chemotherapy. However, in the early period after diagnosis and after 1 year, the survival rates were alike between the cachexia and non-cachexia patients. This lack of difference may explain why the analysis yielded insignificant results. Furthermore, the dosage of the chemotherapeutic agents used was adjusted according to each patient’s requirements. As a result, the effects of these dosage changes on the study outcomes were not broadly investigated, contributing to the variation in result outcomes. In contrast, the study by Hou 31 stood out in terms of overall survival because it included hemoglobin as an additional parameter. This inclusion played a significant role in measuring the decline in overall survival among the patients. Hemoglobin and albumin levels have been linked to a significant decrease in the survival rate among patients with pancreatic cancer cachexia after undergoing chemotherapy. 22
Meta-regression was also performed. The absence of significant findings in the meta-regression analysis regarding age indicates that age did not substantially influence the outcome observed across the studies included. Alongside this, the meta-regression analysis between countries revealed a significant difference, with Japan having more studies compared to Taiwan. This discrepancy can be explained by the fact that the majority of the studies included in our analysis were conducted in Japan.
The global prevalence of cachexia, a debilitating condition affecting approximately 1% of the population in industrialized countries (North America, Europe, and Japan), is a growing concern. This translates to around 9 million patients in these regions. 7 A deeper understanding of cachexia’s pathophysiology is crucial for developing combination therapies, which are urgently needed given the high mortality, poor symptom status, and dismal quality of life associated with cachexia. While some studies have suggested that cachexia can be prevented by an oral drug called Anamorelin, it has shown promising results in Japan in patients undergoing treatment for colorectal cancer, small cell lung cancer and gastric cancer.30,33 It modifies nutritional status among patients and proves to be highly effective. 33 Significance of exercise is reducing muscle inflammation and improving cachexic symptoms has also been noted in our extracted studies. 23 Moreover NSAIDs, immunomodulatory agents, for instance thalidomide and cyclooxygenase-2 inhibitors have benefited patients from cancer cachexia. 23 Cachexia along with other factors can be highly complex condition to manage in ongoing chemotherapy of cancer patients but multimodal therapy regimen has shown to alleviate the symptoms. 31
Future research should prioritize conducting RCTs to validate the impact of cachexia on chemotherapy efficacy in pancreatic cancer patients. Investigating various chemotherapeutic regimens will enhance our understanding of optimal treatment strategies for patients with cachexia, for example, a clinical trial comparing GnP and FFX should be done, aiming to investigate differences in treatment failure. Additionally, trials should explore agents that can either prevent or palliate cachexia, potentially improving patient outcomes and treatment success. Such studies are crucial to developing comprehensive management protocols that integrate both anti-cancer therapies and cachexia control.
Strengths and Limitations
To our knowledge, this meta-analysis represents the first attempt to assess the impact of cancer cachexia on first-line chemotherapy in patients with advanced pancreatic cancer. However, it is essential to acknowledge the limitations of this study. Primarily, the majority of the studies included were retrospective and single-centre in nature; therefore, selection and recall biases regarding weight loss in the study may have influenced the precision of the findings. The generalizability of our findings is constrained by the limited availability of detailed chemotherapy regimen and dose data, reported in only 1 study. Additionally, chemotherapy treatment decisions are tailored to individual patient profiles, varying protocols can significantly influence survival prospects. A significant gap in research exists, with a lack of observational studies representing the Americas and other European countries. Addressing this gap is essential to better understand the global burden of cachexia and develop effective treatments across diverse populations. Due to the limited availability of performance status data (eg, ECOG or KPS) in the included studies, we were unable to conduct a comprehensive analysis on this variable. We recognize this as a limitation and recommend that future research explore the relationship between performance status and cachexia to better understand its impact on treatment outcomes. Additionally, the heterogeneity among the studies, variations in patient populations, and differences in treatment protocols could potentially introduce bias and complicate the interpretation of our findings. Hence, future research should focus on prospective, well-designed studies to further elucidate the impact of cachexia and sarcopenia on treatment outcomes in elderly patients with pancreatic cancer. Furthermore, the absence of RCTs in our study warrants careful consideration to minimize bias and effectively assess the factors. Moreover, although it widely explores various outcomes in a substantial number of patients, the disparity in the number of cachexia patients compared to non-cachexia patients may limit the full representation of cachexia in our analysis.
Conclusion
Our meta-analysis concludes that cachexia is significantly associated with lower overall survival, early chemotherapy failure, and higher mortality compared to non-cachexia patients. Although our review includes data primarily from East Asian countries and the UK, suggesting that while results might not be universally generalizable, variations across different regions are likely to be minimal. Therefore, recognizing and addressing cachexia as an important factor in advanced pancreatic cancer management is fundamental for improving treatment effectiveness and enhancing overall survival. Hence, with timely identification, these patients could receive suitable interventions, thereby enhancing their ability to adhere to planned chemotherapy and improving its effectiveness.
Supplemental Material
Supplemental Material for Impact of Cachexia on Chemotherapy Efficacy and Survival in Pancreatic Cancer: A Systematic Review and Meta-Analysis by Muhammad Ashraf Khalil, Syed Ahsan Ali Jafri, Midhat E. Zahra Naqvi, Iman Jauhar, Vijay Kumar, Syed Muhammad Sinaan Ali, Sara Khalil, Abdul Raheem, Ritesh kumar, Abdul Haseeb, and Muhammad Ashir Shafique in Cancer Control
Supplemental Material for Impact of Cachexia on Chemotherapy Efficacy and Survival in Pancreatic Cancer: A Systematic Review and Meta-Analysis by Muhammad Ashraf Khalil, Syed Ahsan Ali Jafri, Midhat E. Zahra Naqvi, Iman Jauhar, Vijay Kumar, Syed Muhammad Sinaan Ali, Sara Khalil, Abdul Raheem, Ritesh kumar, Abdul Haseeb, and Muhammad Ashir Shafique in Cancer Control
Author Contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Syed Muhammad Sinaan Ali https://orcid.org/0009-0009-8653-7629
Abdul Raheem https://orcid.org/0009-0000-5398-6414
Muhammad Ashir Shafique https://orcid.org/0000-0001-7420-1292
Data Availability Statement
Data available within the article. The authors confirm that the data supporting the findings of this study are available within the article.*
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913. doi: 10.1158/0008-5472.Can-14-0155 [DOI] [PubMed] [Google Scholar]
- 2.Pedrazzoli S. Surgical treatment of pancreatic cancer: currently debated topics on vascular resection. Cancer Control. 2023;30:10732748231153094. doi: 10.1177/10732748231153094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Zhuo Q, Li B, et al. Feasibility of laparoscopic versus open pancreatoduodenectomy following neoadjuvant chemotherapy for borderline resectable pancreatic cancer: a retrospective cohort study. World J Surg Oncol. 2024;22(1):1. doi: 10.1186/s12957-023-03277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabłońska B, Mrowiec S. Pancreatectomy and pancreatic surgery. Life. 2023;13(6):1400. doi: 10.3390/life13061400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 6.Kodama T, Imajima T, Shimokawa M, et al. A multicenter retrospective observational NAPOLEON2 study of nanoliposomal irinotecan with fluorouracil and folinic acid in patients with unresectable pancreatic cancer. Sci Rep. 2024;14(1):12422. doi: 10.1038/s41598-024-63172-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichetti F, Rota S, Ambrosini P, et al. NALIRIFOX, FOLFIRINOX, and gemcitabine with nab-paclitaxel as first-line chemotherapy for metastatic pancreatic cancer: a systematic review and meta-analysis. JAMA Netw Open. 2024;7(1):e2350756. doi: 10.1001/jamanetworkopen.2023.50756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlick K, Gantschnigg A, Seymer A, Huemer F, Greil R, Weiss L. Comparison of gemcitabine plus oxaliplatin versus gemcitabine plus nab-paclitaxel as first-line chemotherapy for advanced pancreatic adenocarcinoma: a single-center retrospective analysis. Cancer Med. 2023;12(16):16997-17004. doi: 10.1002/cam4.6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshmuminov D, Aminjonov B, Palm RF, et al. FOLFIRINOX or gemcitabine-based chemotherapy for borderline resectable and locally advanced pancreatic cancer: a multi-institutional, patient-level, meta-analysis and systematic review. Ann Surg Oncol. 2023;30(7):4417-4428. doi: 10.1245/s10434-023-13353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. 2021;22(16):8491. doi: 10.3390/ijms22168491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argilés JM, López-Soriano FJ, Stemmler B, Busquets S. Cancer-associated cachexia - understanding the tumour macroenvironment and microenvironment to improve management. Nat Rev Clin Oncol. 2023;20(4):250-264. doi: 10.1038/s41571-023-00734-5 [DOI] [PubMed] [Google Scholar]
- 13.Mitsunaga S, Kasamatsu E, Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer. 2020;28(11):5271-5279. doi: 10.1007/s00520-020-05346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kordes M, Larsson L, Engstrand L, Löhr JM. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br J Cancer. 2021;124(10):1623-1636. doi: 10.1038/s41416-021-01301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meza-Valderrama D, Marco E, Dávalos-Yerovi V, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. 2021;13(3):761. doi: 10.3390/nu13030761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543. doi: 10.1007/s00198-009-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson L, Degens H, Li M, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99(1):427-511. doi: 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan AM, Sullivan ES. Impact of musculoskeletal degradation on cancer outcomes and strategies for management in clinical practice. Proc Nutr Soc. 2021;80(1):1-19. doi: 10.1017/s0029665120007855 [DOI] [PubMed] [Google Scholar]
- 19.Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619-635. doi: 10.1002/jcsm.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao WC, Chen PR, Huang CC, et al. Relationship between pancreatic cancer-associated diabetes and cachexia. J Cachexia Sarcopenia Muscle. 2020;11(4):899-908. doi: 10.1002/jcsm.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. 2021;13(6):1980. doi: 10.3390/nu13061980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg. 2018;59:19-26. doi: 10.1016/j.ijsu.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 23.Wong HCY, Lam KY, Chong CCN, Chan AWH, Chan SL. Impact of weight loss during chemotherapy in Chinese patients with unresectable pancreatic cancer. Nutr Cancer. 2019;71(6):954-970. doi: 10.1080/01635581.2019.1595047 [DOI] [PubMed] [Google Scholar]
- 24.Hosokawa K, Nishida T, Hayashi D, et al. Impact of initial body weight loss on prognosis in advanced pancreatic cancer: insights from a single-center retrospective study. Cancer Control. 2023;30:10732748231204719. doi: 10.1177/10732748231204719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan H, Lan J, Bei H, Xu G. The impact of sarcopenia on prognosis of patients with pancreatic cancer: a systematic review and meta-analysis. Scot Med J. 2023;68(4):133-148. doi: 10.1177/00369330231187655 [DOI] [PubMed] [Google Scholar]
- 26.Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156(8):787-788. doi: 10.1001/jamasurg.2021.0522 [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook DA, Reed DA. Appraising the quality of medical education research methods: the medical education research study quality instrument and the newcastle-ottawa scale-education. Acad Med. 2015;90(8):1067. doi: 10.1097/acm.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 29.Carnie L, Abraham M, McNamara MG, Hubner RA, Valle JW, Lamarca A. Impact on prognosis of early weight loss during palliative chemotherapy in patients diagnosed with advanced pancreatic cancer. Pancreatology. 2020;20(8):1682-1688. doi: 10.1016/j.pan.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, Sasaki T, Suzumori C, et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol. 2021;26(7):1293-1303. doi: 10.1007/s10147-021-01912-0 [DOI] [PubMed] [Google Scholar]
- 31.Hou YC, Chen CY, Huang CJ, et al. The differential clinical impacts of cachexia and sarcopenia on the prognosis of advanced pancreatic cancer. Cancers. 2022;14(13):3137. doi: 10.3390/cancers14133137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Saito K, Nakai Y, et al. Early skeletal muscle mass decline is a prognostic factor in patients receiving gemcitabine plus nab-paclitaxel for unresectable pancreatic cancer: a retrospective observational study. Support Care Cancer. 2023;31(3):197. doi: 10.1007/s00520-023-07659-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuse J, Osugi F, Machii K, Niibe K, Endo T. Effect of cancer cachexia on first-line chemotherapy in patients with advanced pancreatic cancer: a claims database study in Japan. Int J Clin Oncol. 2024;29(4):456-463. doi: 10.1007/s10147-024-02467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489. doi: 10.1016/s1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 35.Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154. doi: 10.1016/j.clnu.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 36.Brebach R, Sharpe L, Costa DS, Rhodes P, Butow P. Psychological intervention targeting distress for cancer patients: a meta-analytic study investigating uptake and adherence. Psycho Oncol. 2016;25(8):882. doi: 10.1002/pon.4099 [DOI] [PubMed] [Google Scholar]
- 37.Tao WW, Jiang P, Liu Y, Aungsuroch Y, Tao XM. Psycho-oncologic interventions to reduce distress in cancer patients: a meta-analysis of controlled clinical studies published in People's Republic of China. Psycho Oncol. 2015;24(3):269. doi: 10.1002/pon.3634 [DOI] [PubMed] [Google Scholar]
- 38.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5(2):e200. doi: 10.1038/oncsis.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui JA, Pothuraju R, Jain M, Batra SK, Nasser MW. Advances in cancer cachexia: intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188359. doi: 10.1016/j.bbcan.2020.188359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10(1):140-154. doi: 10.1002/jcsm.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Impact of Cachexia on Chemotherapy Efficacy and Survival in Pancreatic Cancer: A Systematic Review and Meta-Analysis by Muhammad Ashraf Khalil, Syed Ahsan Ali Jafri, Midhat E. Zahra Naqvi, Iman Jauhar, Vijay Kumar, Syed Muhammad Sinaan Ali, Sara Khalil, Abdul Raheem, Ritesh kumar, Abdul Haseeb, and Muhammad Ashir Shafique in Cancer Control
Supplemental Material for Impact of Cachexia on Chemotherapy Efficacy and Survival in Pancreatic Cancer: A Systematic Review and Meta-Analysis by Muhammad Ashraf Khalil, Syed Ahsan Ali Jafri, Midhat E. Zahra Naqvi, Iman Jauhar, Vijay Kumar, Syed Muhammad Sinaan Ali, Sara Khalil, Abdul Raheem, Ritesh kumar, Abdul Haseeb, and Muhammad Ashir Shafique in Cancer Control
Data Availability Statement
Data available within the article. The authors confirm that the data supporting the findings of this study are available within the article.*