Abstract
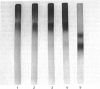
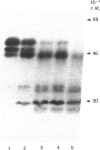
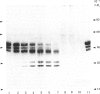
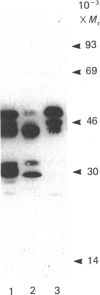
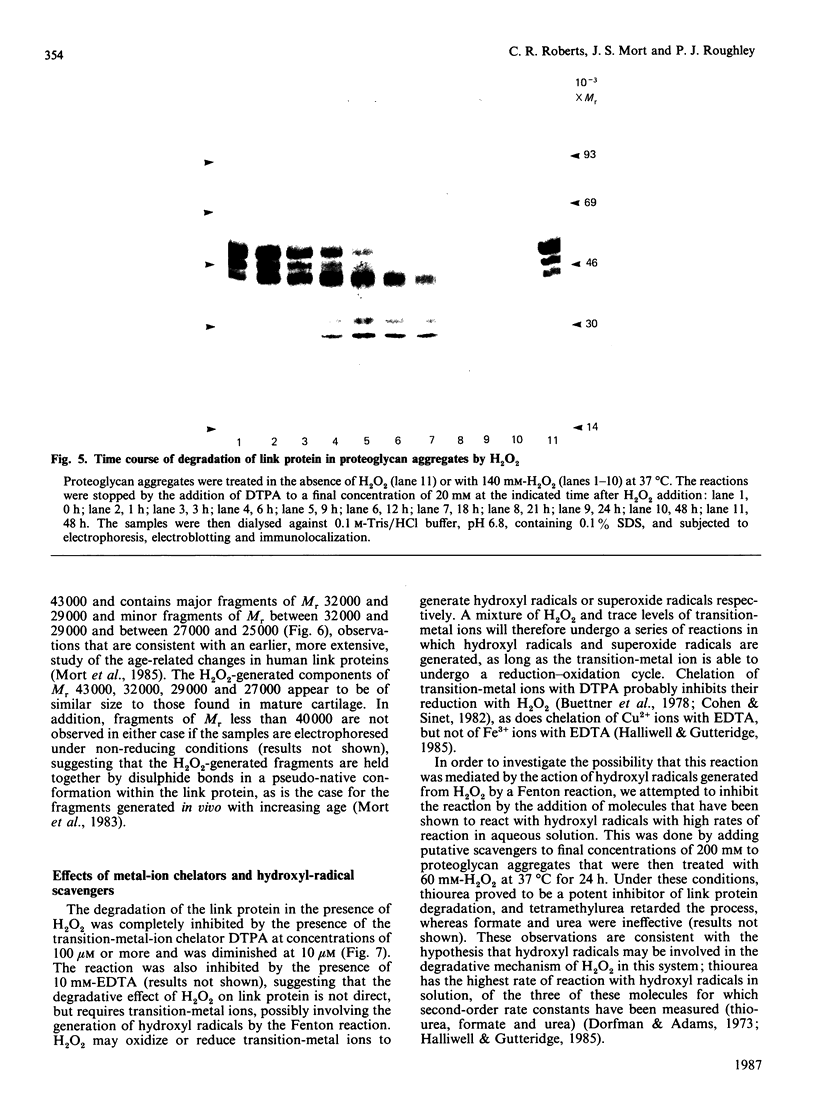
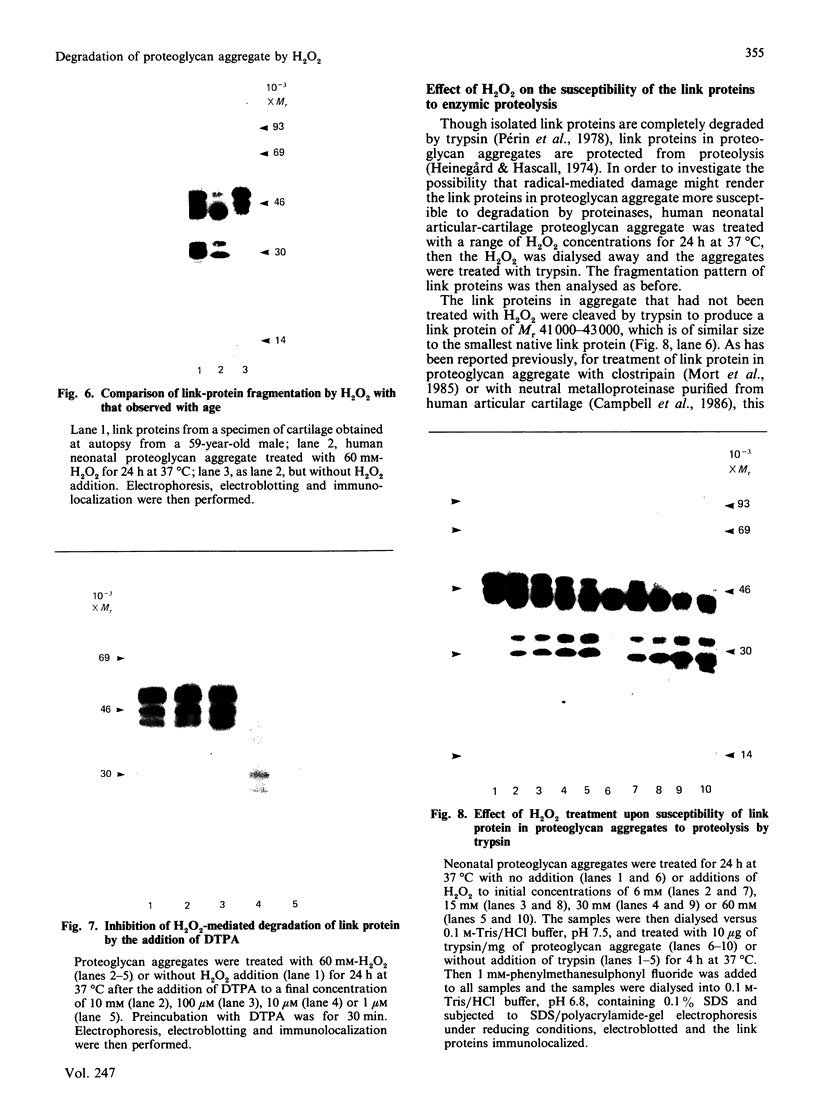
The effects of treatment of purified neonatal human articular-cartilage proteoglycan aggregate with H2O2 were studied. (1) Exposure of proteoglycan aggregate to H2O2 resulted in depolymerization of the aggregate and modification of the core protein of both the proteoglycan subunits and the link proteins. (2) Treatment of the proteoglycan aggregate with H2O2 rendered the proteoglycan subunits unable to interact with hyaluronic acid, with minimal change in their hydrodynamic size. (3) Specific cleavages of the neonatal link proteins occurred. The order in which the major products were generated and their electrophoretic mobilities resembled the pattern observed during human aging. (4) The proteolytic changes in the link proteins were inhibited in the presence of transition-metal-ion chelators, thiourea or tetramethylurea, suggesting that generation of hydroxyl radicals from H2O2 by trace transition-metal ions via a site-specific Fenton reaction may be responsible for the selective cleavages observed. (5) Cleavage of the link proteins in proteoglycan aggregates by H2O2 was shown to have a limited effect on the susceptibility of these proteins to cleavage by trypsin. (6) The relationship between these changes and those observed in cartilage during human aging suggests that some of the age-related changes in the structure of human cartilage proteoglycan aggregate may be the result of radical-mediated damage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartold P. M., Wiebkin O. W., Thonard J. C. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontal Res. 1984 Jul;19(4):390–400. doi: 10.1111/j.1600-0765.1984.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Harper G. S., Lowther D. A., Preston B. N. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984 May;8(5):629–637. [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W., Leuthauser S. W. The effect of iron on the distribution of superoxide and hydroxyl radicals as seen by spin trapping and on the superoxide dismutase assay. Photochem Photobiol. 1978 Oct-Nov;28(4-5):693–695. doi: 10.1111/j.1751-1097.1978.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Christner J. E., Lee Y., Lentz M. Monoclonal antibodies as probes for determining the microheterogeneity of the link proteins of cartilage proteoglycan. J Biol Chem. 1985 Sep 15;260(20):11348–11356. [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kesner L., Chan P. C. Degradation of articular cartilage by copper and hydrogen peroxide. Agents Actions. 1984 Oct;15(3-4):328–335. doi: 10.1007/BF01972367. [DOI] [PubMed] [Google Scholar]
- Cooper B., Creeth J. M., Donald A. S. Studies of the limited degradation of mucus glycoproteins. The mechanism of the peroxide reaction. Biochem J. 1985 Jun 15;228(3):615–626. doi: 10.1042/bj2280615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Forni L. G. Oxygen-centred free radicals can efficiently degrade the polypeptide of proteoglycans in whole cartilage. Biosci Rep. 1984 Dec;4(12):1017–1026. doi: 10.1007/BF01116694. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Jessup W. Fragmentation of extracellular and intracellular polypeptides by free radicals. Prog Clin Biol Res. 1985;180:341–350. [PubMed] [Google Scholar]
- Fligiel S. E., Lee E. C., McCoy J. P., Johnson K. J., Varani J. Protein degradation following treatment with hydrogen peroxide. Am J Pathol. 1984 Jun;115(3):418–425. [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- LABELLA F. S. STRUCTURE OF COLLAGEN FROM HUMAN TENDON AS INFLUENCED BY AGE AND SEX. J Gerontol. 1965 Jan;20:54–59. doi: 10.1093/geronj/20.1.54. [DOI] [PubMed] [Google Scholar]
- LaBella F. S., Vivian S., Thornhill D. P. Amino acid composition of human aortic elastin as influenced by age. J Gerontol. 1966 Oct;21(4):550–555. doi: 10.1093/geronj/21.4.550. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Caterson B., Poole A. R., Roughley P. J. The origin of human cartilage proteoglycan link-protein heterogeneity and fragmentation during aging. Biochem J. 1985 Dec 15;232(3):805–812. doi: 10.1042/bj2320805. [DOI] [PMC free article] [PubMed] [Google Scholar]
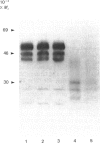
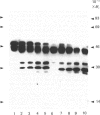
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Mar 15;261(8):3519–3535. [PubMed] [Google Scholar]
- Niedermeier W., Griggs J. H. Trace metal composition of synovial fluid and blood serum of patients with rheumatoid arthritis. J Chronic Dis. 1971 Jan;23(8):527–536. doi: 10.1016/0021-9681(71)90128-7. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. The formation of free radicals and the consequences of their reactions in vivo. Photochem Photobiol. 1978 Oct-Nov;28(4-5):787–801. doi: 10.1111/j.1751-1097.1978.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Jollés P. The action of trypsin on purified link proteins from bovine nasal cartilage proteoglycan complex. FEBS Lett. 1978 Oct 15;94(2):257–260. doi: 10.1016/0014-5793(78)80950-8. [DOI] [PubMed] [Google Scholar]
- Rivett A. J., Roseman J. E., Oliver C. N., Levine R. L., Stadtman E. R. Covalent modification of proteins by mixed-function oxidation: recognition by intracellular proteases. Prog Clin Biol Res. 1985;180:317–328. [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Scott J. E., Tigwell M. J., Sajdera S. W. Loss of basophilic (sulphated) material from sections of cartilage treated with periodate solution. Histochem J. 1972 Mar;4(2):155–167. doi: 10.1007/BF01004974. [DOI] [PubMed] [Google Scholar]
- Scudder P. R., McMurray W., White A. G., Dormandy T. L. Synovial fluid copper and related variables in rheumatoid and degenerative arthritis. Ann Rheum Dis. 1978 Feb;37(1):71–72. doi: 10.1136/ard.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senator G. B., Muirden K. D. Concentration of iron in synovial membrane, synovial fluid, and serum in rheumatoid arthritis and other joint diseases. Ann Rheum Dis. 1968 Jan;27(1):49–54. doi: 10.1136/ard.27.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. F., Halliwell B., Richmond R., Skowroneck W. R. The role of superoxide and hydroxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J Inorg Biochem. 1981 Apr;14(2):127–134. doi: 10.1016/s0162-0134(00)80033-1. [DOI] [PubMed] [Google Scholar]