Abstract
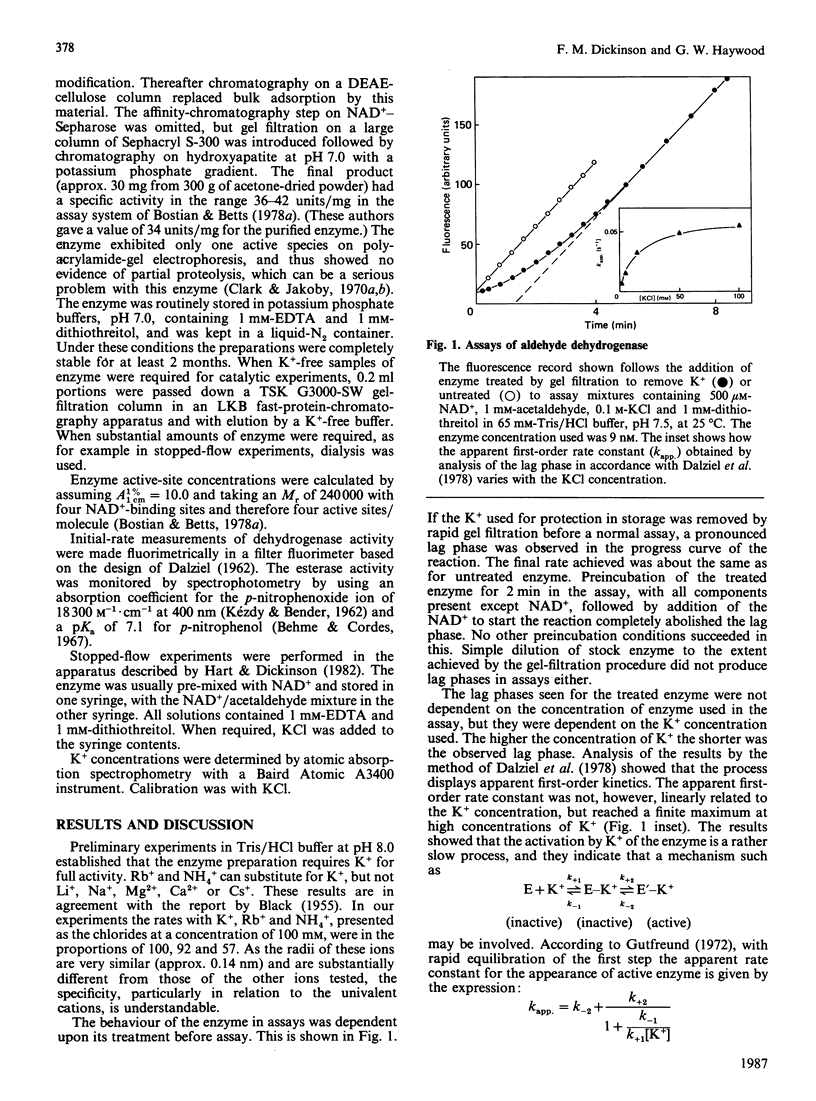
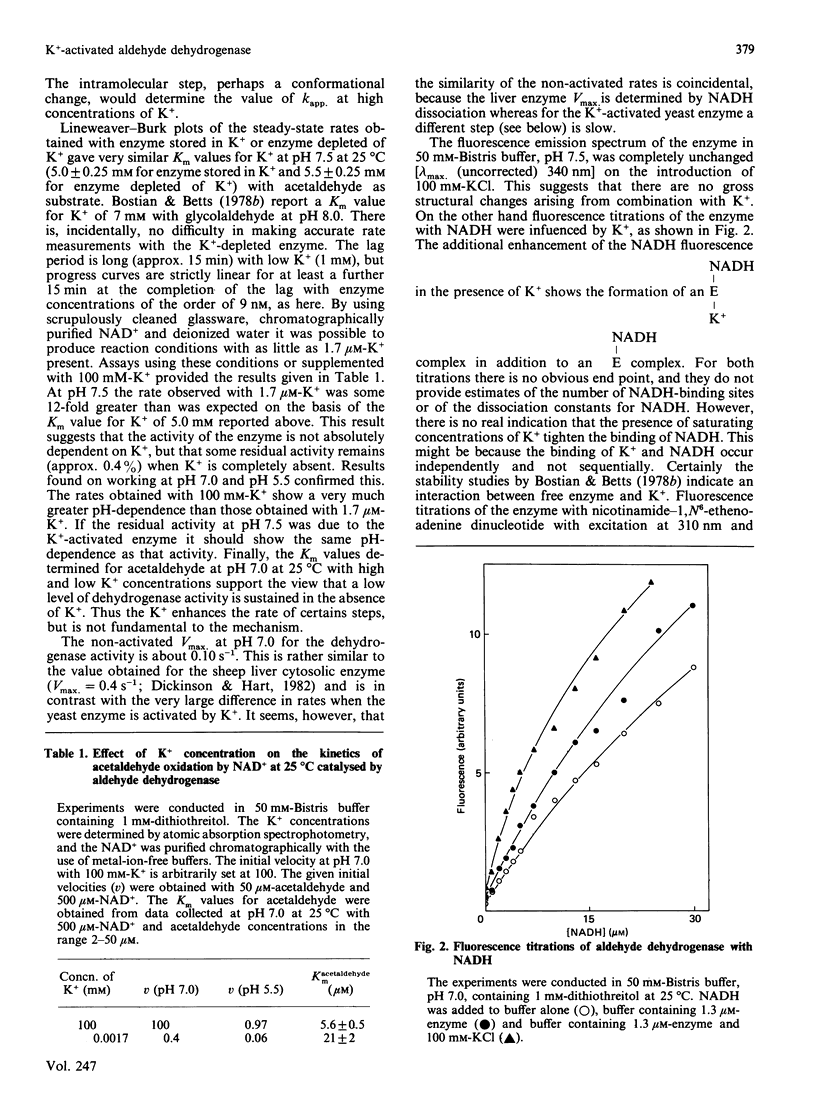
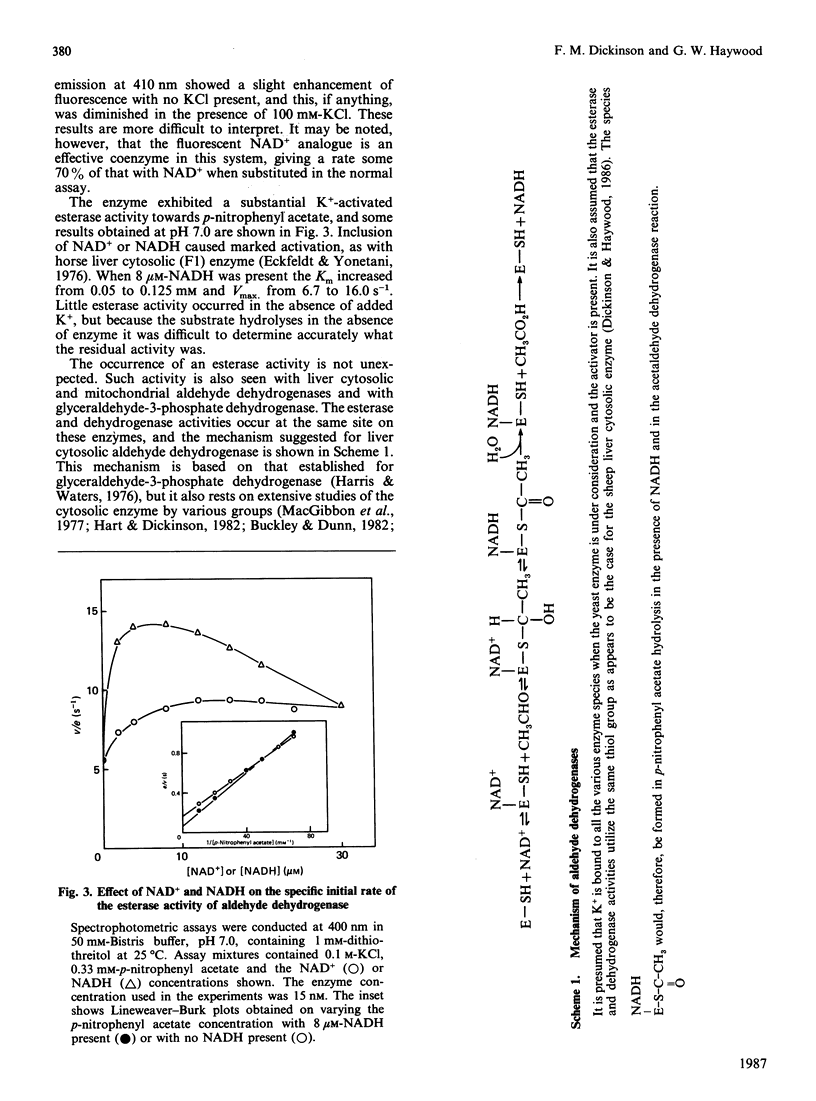
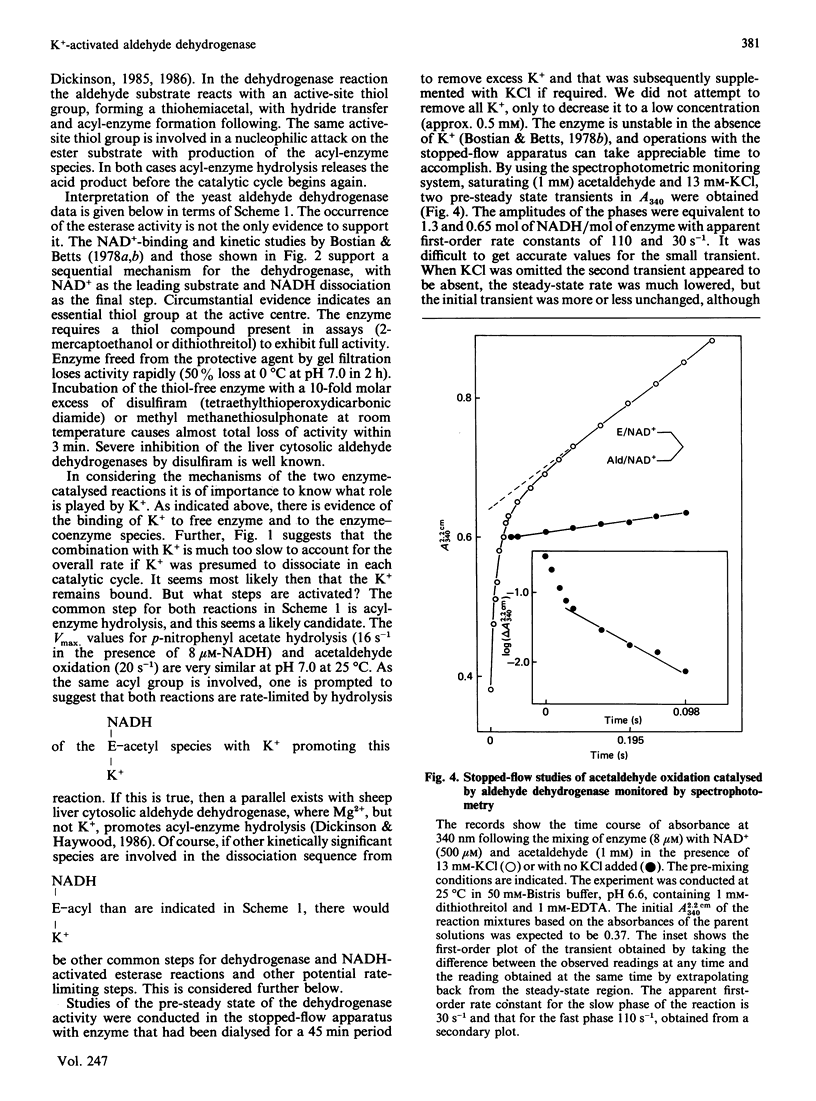
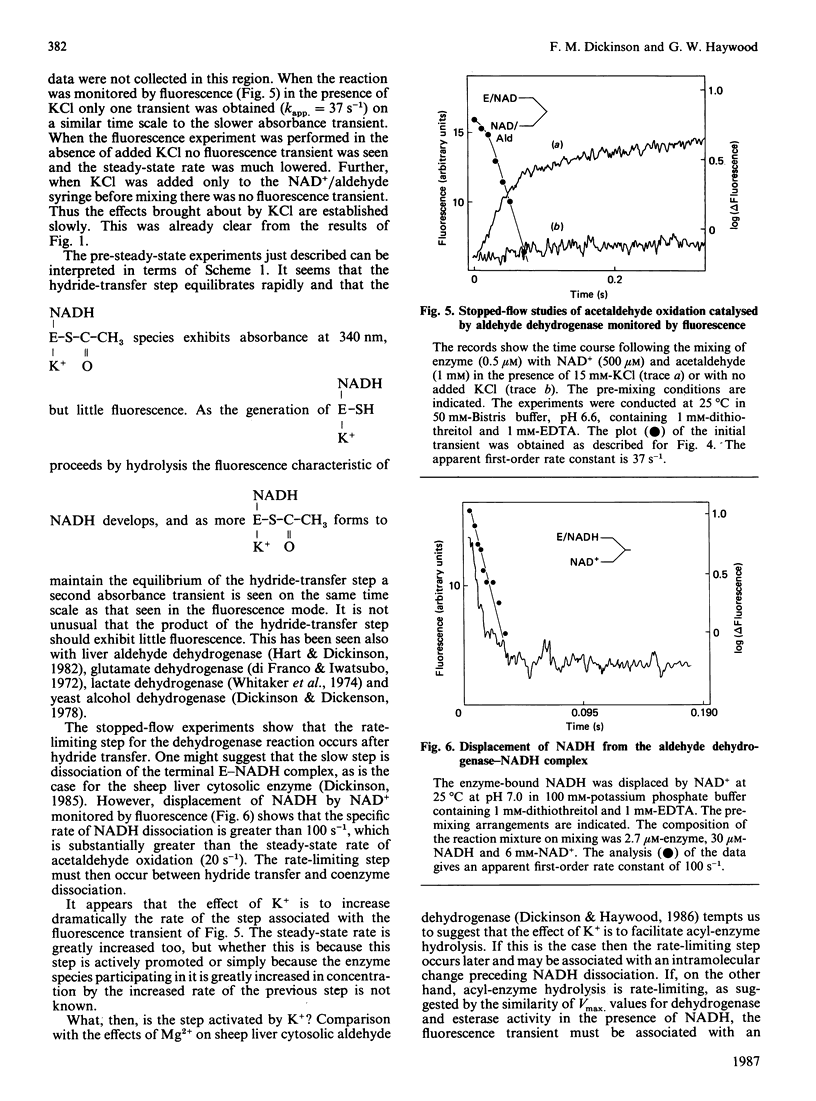
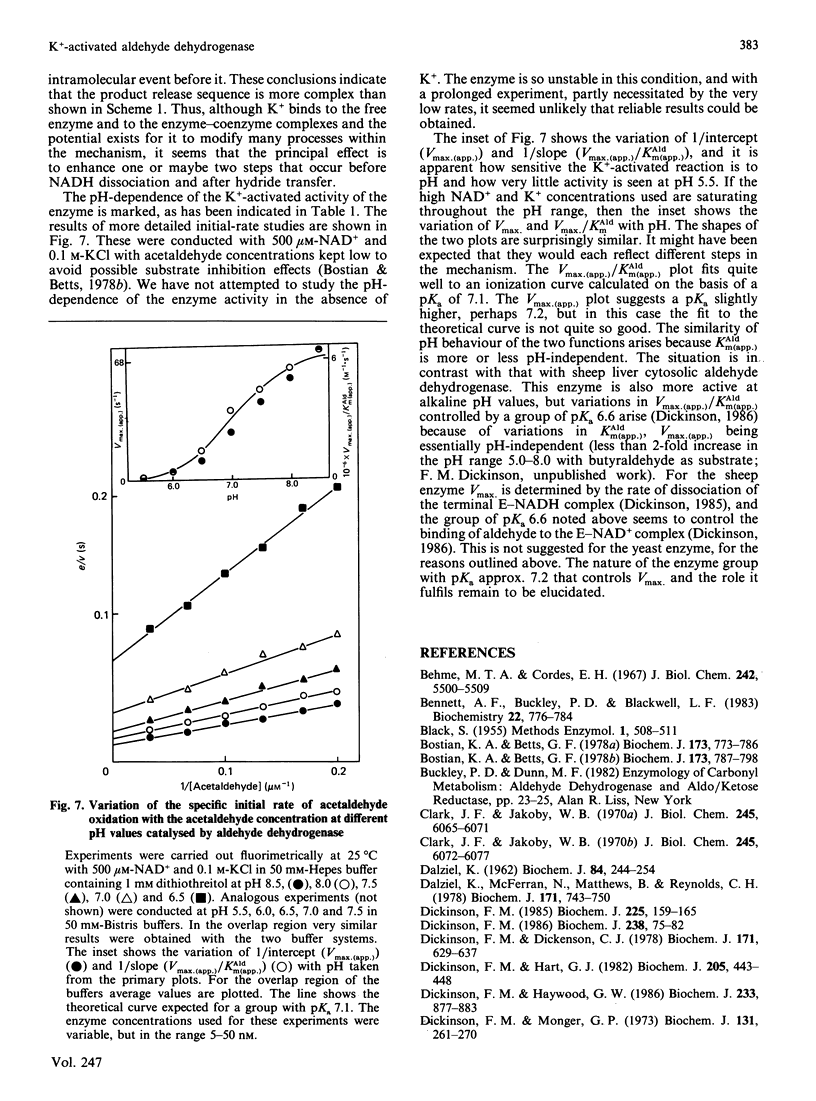
The effect of K+ on assays of the enzyme was studied and it appears that the activation occurs slowly by a two-step process. Kinetic measurements suggest that the enzyme-catalysed reaction can proceed slowly (0.4%) in the complete absence of K+. The enzyme exhibits a K+-activated esterase activity, which is further activated by NAD+ or NADH. Stopped-flow studies indicated that the principal effect of K+ on the dehydrogenase reaction is to accelerate a step (possibly acyl-enzyme hydrolysis) associated with a fluorescence and small absorbance transient that occurs after hydride transfer and before NADH dissociation from the terminal E-NADH complex. The variation of activity of the enzyme with pH was studied. An enzyme group with pKa approx. 7.1 apparently promotes enzyme activity when in its alkaline form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behme M. T., Cordes E. H. Kinetics of the glyceraldehyde 3-phosphate dehydrogenase-catalyzed hydrolysis of p-nitrophenyl acetate. J Biol Chem. 1967 Dec 10;242(23):5500–5509. [PubMed] [Google Scholar]
- Bennett A. F., Buckley P. D., Blackwell L. F. Inhibition of the dehydrogenase activity of sheep liver cytoplasmic aldehyde dehydrogenase by magnesium ions. Biochemistry. 1983 Feb 15;22(4):776–784. doi: 10.1021/bi00273a011. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Betts G. F. Kinetics and reaction mechanism of potassium-activated aldehyde dehydrogenase from Saccharomyces cerevisiae. Biochem J. 1978 Sep 1;173(3):787–798. doi: 10.1042/bj1730787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Betts G. F. Rapid purification and properties of potassium-activated aldehyde dehydrogenase from Saccharomyces cerevisiae. Biochem J. 1978 Sep 1;173(3):773–786. doi: 10.1042/bj1730773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. F., Jakoby W. B. Yeast aldehyde dehydrogenase. 3. Preparation of three homogeneous species. J Biol Chem. 1970 Nov 25;245(22):6065–6071. [PubMed] [Google Scholar]
- Clark J. F., Jakoby W. B. Yeast aldehyde dehydrogenase. IV. Dissociation and reassociation of native and hybrid forms. J Biol Chem. 1970 Nov 25;245(22):6072–6077. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., McFerran N., Matthews B., Reynolds C. H. Transient kinetics of nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase from bovine heart mitochondria. Biochem J. 1978 Jun 1;171(3):743–750. doi: 10.1042/bj1710743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Franco A., Iwatsubo M. Reaction mechanism of L-glutamate dehydrogenase. Characterization of optical and kinetic properties of various enzyme-reduced-coenzyme complexes. Eur J Biochem. 1972 Nov 7;30(3):517–532. doi: 10.1111/j.1432-1033.1972.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Dickenson C. J. Estimation of rate and dissociation constants involving ternary complexes in reactions catalysed by yeast alcohol dehydrogenase. Biochem J. 1978 Jun 1;171(3):629–637. doi: 10.1042/bj1710629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J. Effects of Mg2+, Ca2+ and Mn2+ on sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Aug 1;205(2):443–448. doi: 10.1042/bj2050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Haywood G. W. The effects of Mg2+ on certain steps in the mechanisms of the dehydrogenase and esterase reactions catalysed by sheep liver aldehyde dehydrogenase. Support for the view that dehydrogenase and esterase activities occur at the same site on the enzyme. Biochem J. 1986 Feb 1;233(3):877–883. doi: 10.1042/bj2330877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Studies on the mechanism of sheep liver cytosolic aldehyde dehydrogenase. The effect of pH on the aldehyde binding reactions and a re-examination of the problem of the site of proton release in the mechanism. Biochem J. 1986 Aug 15;238(1):75–82. doi: 10.1042/bj2380075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Studies on the mechanism of sheep liver cytosolic aldehyde dehydrogenase. Biochem J. 1985 Jan 1;225(1):159–165. doi: 10.1042/bj2250159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckfeldt J. H., Yonetani T. Kinetics and mechanism of the F1 isozyme of horse liver aldehyde dehydrogenase. Arch Biochem Biophys. 1976 Mar;173(1):273–281. doi: 10.1016/0003-9861(76)90260-5. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Kinetic properties of highly purified preparations of sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Jun 1;203(3):617–627. doi: 10.1042/bj2030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- MacGibbon A. K., Blackwell L. F., Buckley P. D. Kinetics of sheep-liver cytoplasmic aldehyde dehydrogenase. Eur J Biochem. 1977 Jul 1;77(1):93–100. doi: 10.1111/j.1432-1033.1977.tb11645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]


