Abstract
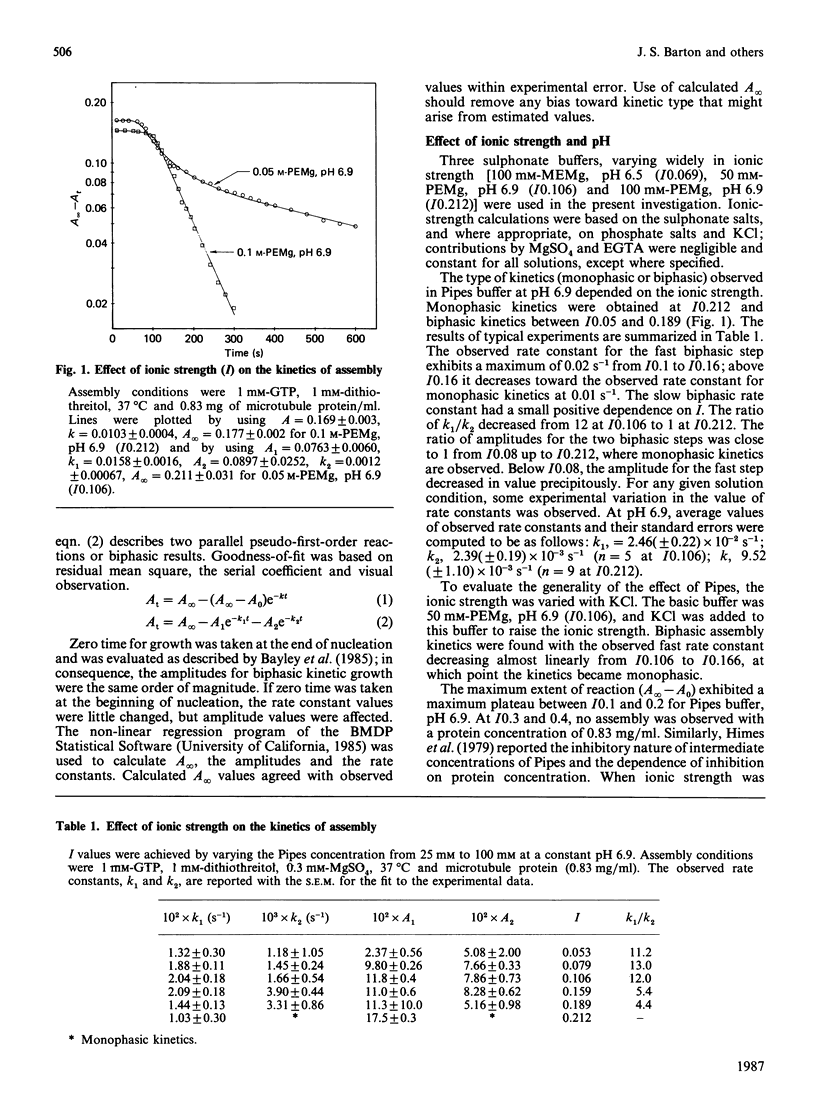
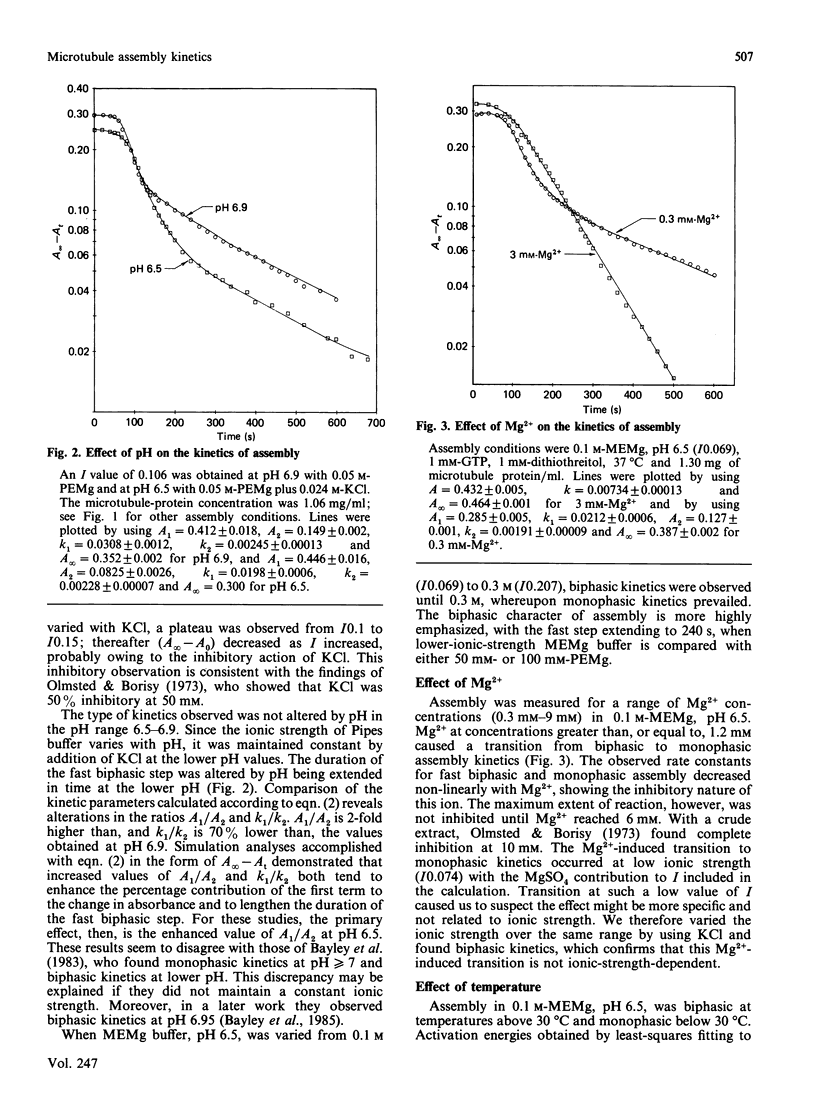
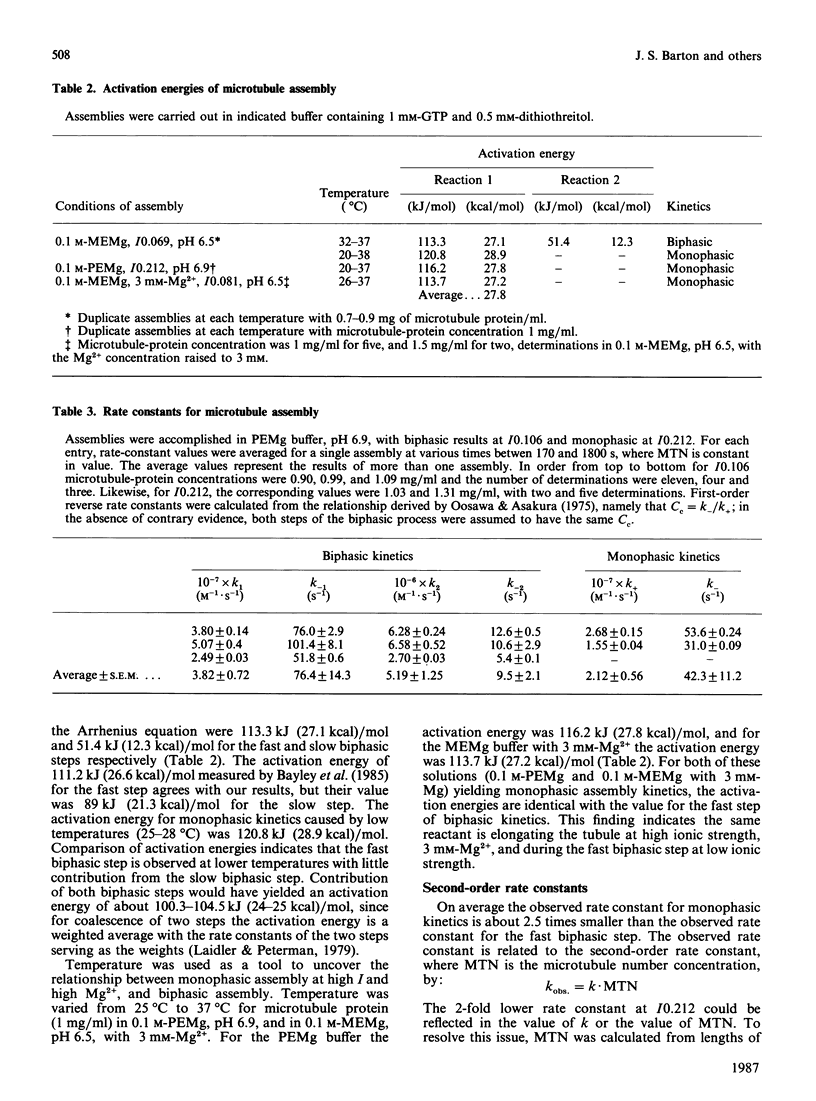
The assembly kinetics of microtubule protein are altered by ionic strength, temperature and Mg2+, but not by pH. High ionic strength (I0.2), low temperature (T less than 30 degrees C) and elevated Mg2+ (greater than or equal to 1.2 mM) induce a transition from biphasic to monophasic kinetics. Comparison of the activation energy obtained for the fast biphasic step at low ionic strength (I0.069) shows excellent agreement with the values obtained at high ionic strength, low temperature and elevated Mg2+. From this observation it can be implied that the tubulin-containing reactant of the fast biphasic event is also the species that elongates microtubules during monophasic assembly. Second-order rate constants for biphasic assembly are 3.82(+/- 0.72) x 10(7) M-1.s-1 and 5.19(+/- 1.25) x 10(6) M-1.s-1, and for monophasic assembly the rate constant is 2.12(+/- 0.56) x 10(7) M-1.s-1. The microtubule number concentration is constant during elongation of microtubules for biphasic and monophasic assembly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. S., Riazi G. H. Evidence against tubulin oligomer dissociation to tubulin dimer at assembly temperatures. Biochim Biophys Acta. 1982 Jul 12;705(1):8–11. doi: 10.1016/0167-4838(82)90328-4. [DOI] [PubMed] [Google Scholar]
- Barton J. S., Riazi G. H. Evidence for two growth steps in microtubule polymerization. Biochim Biophys Acta. 1980 Jul 3;630(3):392–401. doi: 10.1016/0304-4165(80)90288-3. [DOI] [PubMed] [Google Scholar]
- Bayley P. M., Butler F. M., Clark D. C., Manser E. J., Martin S. R. The assembly of microtubule protein in vitro. The kinetic role in microtubule elongation of oligomeric fragments containing microtubule-associated proteins. Biochem J. 1985 Apr 15;227(2):439–455. doi: 10.1042/bj2270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley P. M., Charlwood P. A., Clark D. C., Martin S. R. Oligomeric species in glycerol-cycled bovine-brain microtubule protein. Analytical ultracentrifugal characterisation. Eur J Biochem. 1982 Jan;121(3):579–585. doi: 10.1111/j.1432-1033.1982.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Bayley P. M., Clark D. C., Martin S. R. Conformational properties of microtubule protein: their relation to the self-assembly process in vitro. Biopolymers. 1983 Jan;22(1):87–91. doi: 10.1002/bip.360220114. [DOI] [PubMed] [Google Scholar]
- Bordas J., Mandelkow E. M., Mandelkow E. Stages of tubulin assembly and disassembly studied by time-resolved synchrotron X-ray scattering. J Mol Biol. 1983 Feb 15;164(1):89–135. doi: 10.1016/0022-2836(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryan J. A quantitative analysis of microtubule elongation. J Cell Biol. 1976 Dec;71(3):749–767. doi: 10.1083/jcb.71.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Direct incorporation of microtubule oligomers at high GTP concentrations. FEBS Lett. 1984 Jul 23;173(1):67–74. doi: 10.1016/0014-5793(84)81019-4. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Microtubule surface lattice and subunit structure and observations on reassembly. J Cell Biol. 1974 Jan;60(1):153–167. doi: 10.1083/jcb.60.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell K. W., Jordan M. A. A kinetic analysis of assembly-disassembly at opposite microtubule ends. J Biol Chem. 1982 Mar 25;257(6):3131–3138. [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. II. Thermodynamics. Biochemistry. 1975 Oct 21;14(21):4567–4573. doi: 10.1021/bi00692a002. [DOI] [PubMed] [Google Scholar]
- Gal V., Trajković D., Ristanović D. Double and single exponential kinetics of microtubule assembly in vitro. Int J Biochem. 1986;18(1):85–88. doi: 10.1016/0020-711x(86)90013-3. [DOI] [PubMed] [Google Scholar]
- Himes R. H., Newhouse C. S., Haskins K. M., Burton P. R. Effects of sulfonate anions on the self-assembly of brain tubulin. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1031–1038. doi: 10.1016/s0006-291x(79)80011-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C., Weingarten M., Gerhart J. C. Microtubules from mammalian brain: some properties of their depolymerization products and a proposed mechanism of assembly and disassembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1159–1163. doi: 10.1073/pnas.71.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. Taxol-induced polymerization of purified tubulin. Mechanism of action. J Biol Chem. 1981 Oct 25;256(20):10435–10441. [PubMed] [Google Scholar]
- Laidler K. J., Peterman B. F. Temperature effects in enzyme kinetics. Methods Enzymol. 1979;63:234–257. doi: 10.1016/0076-6879(79)63012-4. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Harmsen A., Mandelkow E., Bordas J. X-ray kinetic studies of microtubule assembly using synchrotron radiation. Nature. 1980 Oct 16;287(5783):595–599. doi: 10.1038/287595a0. [DOI] [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M., Bordas J. Structure of tubulin rings studied by X-ray scattering using synchrotron radiation. J Mol Biol. 1983 Jun 15;167(1):179–196. doi: 10.1016/s0022-2836(83)80040-0. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Borisy G. G. Characterization of microtubule protein oligomers by analytical ultracentrifugation. J Biol Chem. 1978 Apr 25;253(8):2825–2833. [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F., Simon C., Batelier G. Mechanism of tubulin assembly: role of rings in the nucleation process and of associated proteins in the stabilization of microtubules. Biochemistry. 1981 Aug 4;20(16):4709–4716. doi: 10.1021/bi00519a029. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purich D. L., Kristofferson D. Microtubule assembly: a review of progress, principles, and perspectives. Adv Protein Chem. 1984;36:133–212. doi: 10.1016/s0065-3233(08)60297-1. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Borisy G. G. Electron microscopy of metal-shadowed and negatively stained microtubule protein. Structure of the 30 S oligomer. J Biol Chem. 1978 Apr 25;253(8):2846–2851. [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. Tubulin rings: curved filaments with limited flexibility and two modes of association. J Supramol Struct. 1979;10(4):419–431. doi: 10.1002/jss.400100405. [DOI] [PubMed] [Google Scholar]


