Abstract
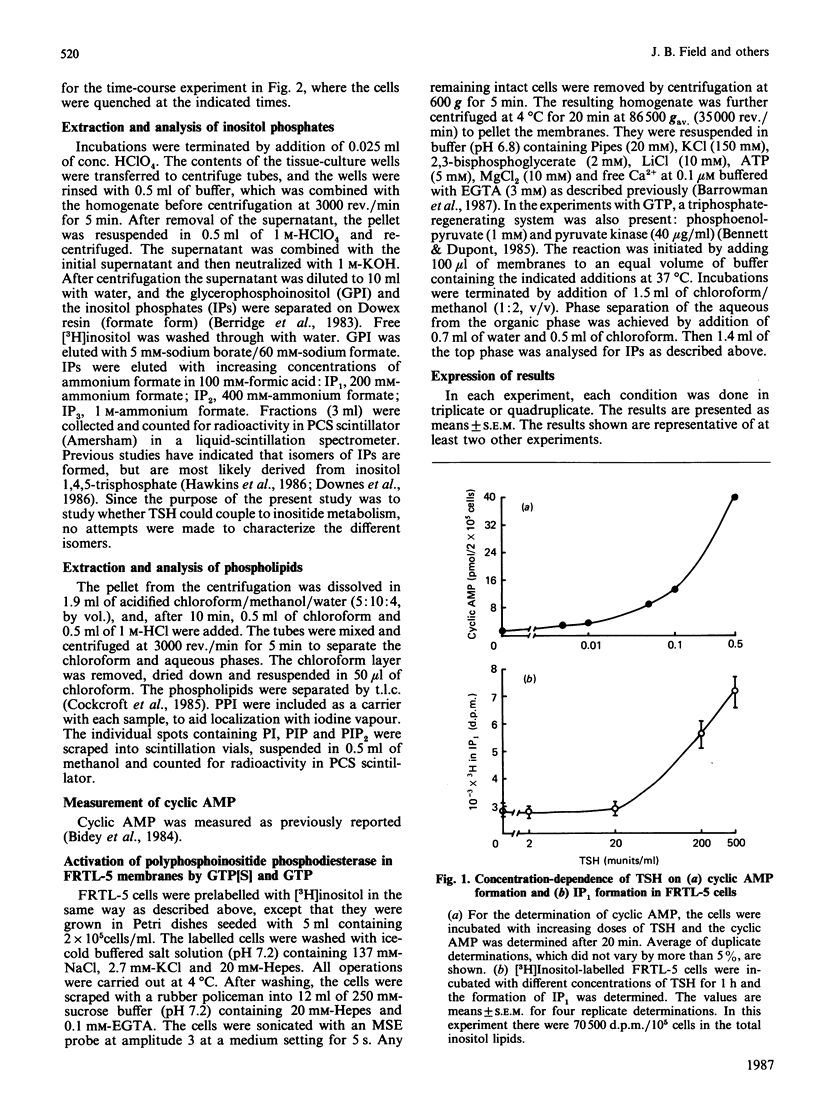
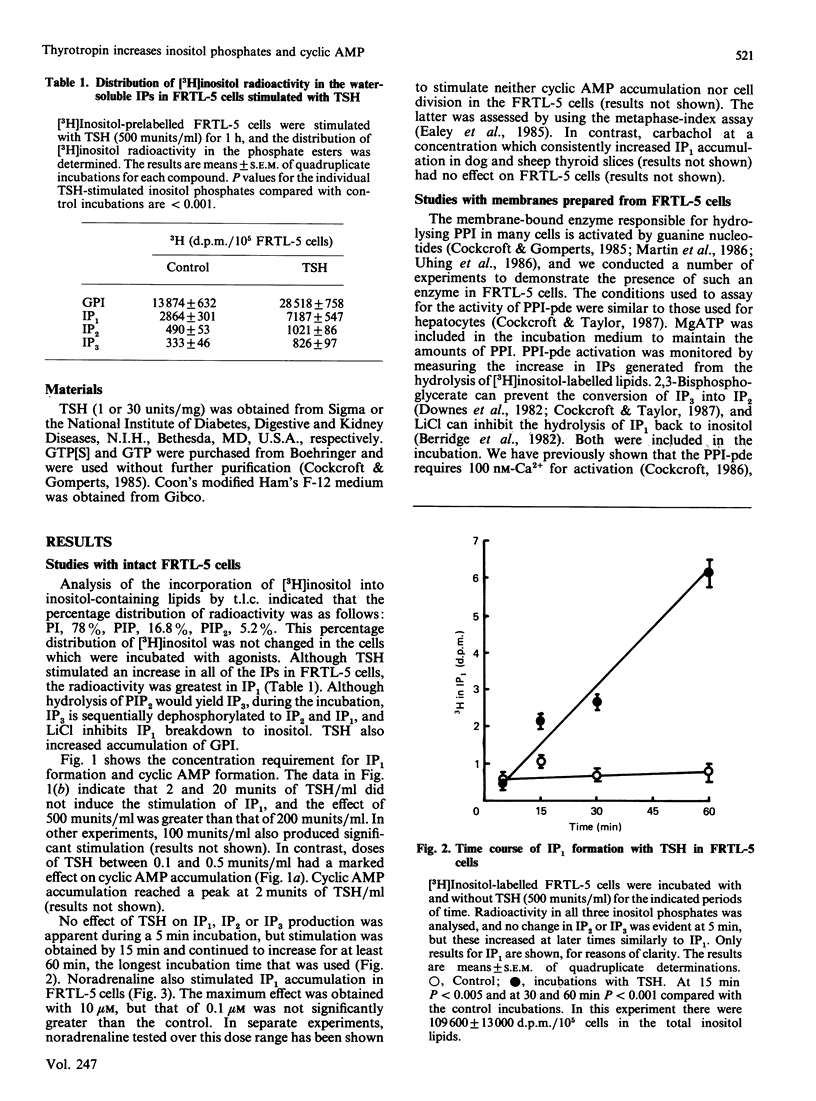
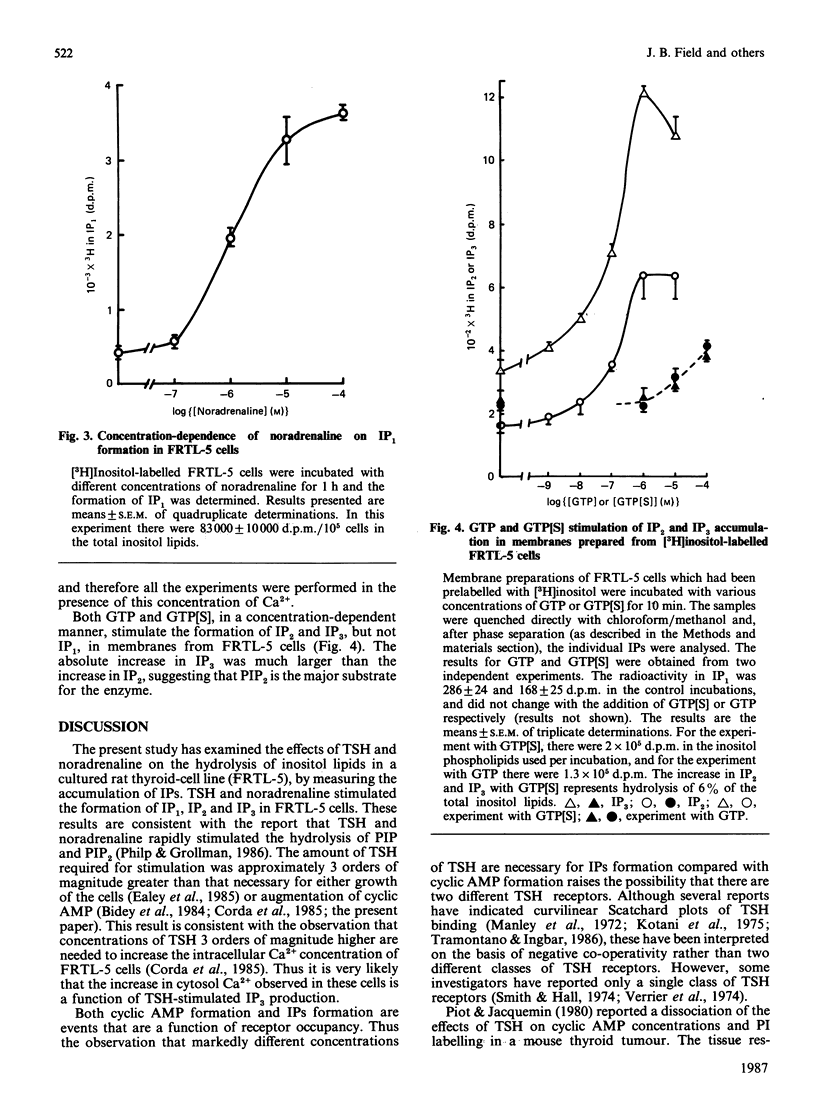
Studies were conducted to determine whether thyroid-stimulating hormone (TSH; thyrotropin), a hormone known to increase cytosol concentrations of cyclic AMP, also stimulates the formation of inositol phosphates in thyroid cells. TSH and noradrenaline both stimulated [3H]inositol phosphate formation in a concentration-dependent manner in the rat thyroid cell line, FRTL-5 cells, which had been prelabelled with [3H]inositol. The threshold concentration of TSH required to stimulate inositol phosphate formation was more than 20 munits/ml, which is approx. 10(3)-fold greater than that required for cyclic AMP accumulation and growth in these cells. We also demonstrate that membranes prepared from FRTL-5 cells possess a guanine nucleotide-activatable polyphosphoinositide phosphodiesterase, which suggests that activation of inositide metabolism in these cells may be coupled to receptors by the G-protein, Gp. Our findings suggest that two second-messenger systems exist to mediate the action of TSH in the thyroid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Ernst Klenk Lecture, November 1985. Intracellular signalling through inositol trisphosphate and diacylglycerol. Biol Chem Hoppe Seyler. 1986 Jun;367(6):447–456. [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P. Separate 5-hydroxytryptamine receptors on the salivary gland of the blowfly are linked to the generation of either cyclic adenosine 3',5'-monophosphate or calcium signals. Br J Pharmacol. 1981 Jul;73(3):729–738. doi: 10.1111/j.1476-5381.1981.tb16809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bidey S. P., Chiovato L., Day A., Turmaine M., Gould R. P., Ekins R. P., Marshall N. J. Evaluation of the rat thyroid cell strain FRTL-5 as an in-vitro bioassay system for thyrotrophin. J Endocrinol. 1984 Jun;101(3):269–276. doi: 10.1677/joe.0.1010269. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A., Judah J. D. Subcellular localisation of inositol lipid kinases in rat liver. Biochim Biophys Acta. 1985 May 30;845(2):163–170. doi: 10.1016/0167-4889(85)90173-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. The dependence on Ca2+ of the guanine-nucleotide-activated polyphosphoinositide phosphodiesterase in neutrophil plasma membranes. Biochem J. 1986 Dec 1;240(2):503–507. doi: 10.1042/bj2400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda D., Marcocci C., Kohn L. D., Axelrod J., Luini A. Association of the changes in cytosolic Ca2+ and iodide efflux induced by thyrotropin and by the stimulation of alpha 1-adrenergic receptors in cultured rat thyroid cells. J Biol Chem. 1985 Aug 5;260(16):9230–9236. [PubMed] [Google Scholar]
- Davis J. S., Weakland L. L., West L. A., Farese R. V. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem J. 1986 Sep 1;238(2):597–604. doi: 10.1042/bj2380597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealey P. A., Emmerson J. M., Bidey S. P., Marshall N. J. Thyrotrophin stimulation of mitogenesis of the rat thyroid cell strain FRTL-5: a metaphase index assay for the detection of thyroid growth stimulators. J Endocrinol. 1985 Aug;106(2):203–210. doi: 10.1677/joe.0.1060203. [DOI] [PubMed] [Google Scholar]
- FREINKEL N. Pathways of thyroidal phosphorus metabolism: the effect of pituitary thyrotropin upon the phospholipids of the sheep thyroid gland. Endocrinology. 1957 Oct;61(4):448–460. doi: 10.1210/endo-61-4-448. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Rosic N., Babischkin J., Farese M. G., Foster R., Davis J. S. Dual activation of the inositol-triphosphate-calcium and cyclic nucleotide intracellular signaling systems by adrenocorticotropin in rat adrenal cells. Biochem Biophys Res Commun. 1986 Mar 28;135(3):742–748. doi: 10.1016/0006-291x(86)90991-5. [DOI] [PubMed] [Google Scholar]
- Field J. B. Thyroid-stimulating hormone and cyclic adenosine 3',5'-monophosphate in the regulation of thyroid gland function. Metabolism. 1975 Mar;24(3):381–393. doi: 10.1016/0026-0495(75)90118-3. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haye B., Jacquemin C. Stimulation par la thyréostimuline de la production de l'inositol 1-2 phosphate cyclique. Biochimie. 1974;56(9):1283–1285. doi: 10.1016/s0300-9084(74)80022-2. [DOI] [PubMed] [Google Scholar]
- Hirayu H., Magnusson R. P., Rapoport B. Studies on the mechanism of desensitization of the cyclic AMP response to TSH stimulation in a cloned rat thyroid cell line. Mol Cell Endocrinol. 1985 Aug;42(1):21–27. doi: 10.1016/0303-7207(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., Kondo Y. Acute effect of thyrotropin on phosphatidylinositol degradation and transient accumulation of diacylglycerol in isolated thyroid follicles. Biochem Biophys Res Commun. 1980 Nov 28;97(2):759–765. doi: 10.1016/0006-291x(80)90329-0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M., Kariya T., Field J. B. Studies of thyroid-stimulating hormone binding to bovine thyroid plasma membranes. Metabolism. 1975 Aug;24(8):959–971. doi: 10.1016/0026-0495(75)90088-8. [DOI] [PubMed] [Google Scholar]
- MORTON M. E., SCHWARTZ J. R. The stimulation in vitro of phospholipid synthesis in thyroid tissue by thyrotrophic hormone. Science. 1953 Jan 30;117(3031):103–104. doi: 10.1126/science.117.3031.103. [DOI] [PubMed] [Google Scholar]
- Manley S. W., Bourke J. R., Hawker R. W. Reversible binding of labelled and non-labelled thyrotrophin by intact thyroid tissue in vitro. J Endocrinol. 1972 Dec;55(3):555–563. doi: 10.1677/joe.0.0550555. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Nambi P., Peters J. R., Sibley D. R., Lefkowitz R. J. Desensitization of the turkey erythrocyte beta-adrenergic receptor in a cell-free system. Evidence that multiple protein kinases can phosphorylate and desensitize the receptor. J Biol Chem. 1985 Feb 25;260(4):2165–2171. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Philp N. J., Grollman E. F. Thyrotropin and norepinephrine stimulate the metabolism of phosphoinositides in FRTL-5 thyroid cells. FEBS Lett. 1986 Jul 7;202(2):193–196. doi: 10.1016/0014-5793(86)80685-8. [DOI] [PubMed] [Google Scholar]
- Piot J. M., Jacquemin C. Lack of adenylate and guanylate cyclases responsiveness to hormones in a spontaneous murine thyroid tumor. Biochem Biophys Res Commun. 1980 Jul 16;95(1):357–366. doi: 10.1016/0006-291x(80)90746-9. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Patel J. Phorbol ester causes desensitization of gonadotropin-responsive adenylate cyclase in a murine Leydig tumor cell line. J Biol Chem. 1985 Jul 5;260(13):8026–8031. [PubMed] [Google Scholar]
- Scott T. W., Freinkel N., Klein J. H., Nitzan M. Metabolism of phospholipids, neutral lipids and carbohydrates in dispersed porcine thyroid cells: comparative effects of pituitary thyrotropin and dibutyryl-3',5'-adenosine monophosphate on the turnover of slices from pig thyroid. Endocrinology. 1970 Nov;87(5):854–863. doi: 10.1210/endo-87-5-854. [DOI] [PubMed] [Google Scholar]
- Shuman S. J., Zor U., Chayoth R., Field J. B. Exposure of thyroid slices to thyroid-stimulating hormone induces refractoriness of the cyclic AMP system to subsequent hormone stimulation. J Clin Invest. 1976 May;57(5):1132–1141. doi: 10.1172/JCI108380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Binding of thyroid stimulators to thyroid membranes. FEBS Lett. 1974 Jun 15;42(3):301–304. doi: 10.1016/0014-5793(74)80751-9. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Ingbar S. H. Properties and regulation of the thyrotropin receptor in the FRTL5 rat thyroid cell line. Endocrinology. 1986 May;118(5):1945–1951. doi: 10.1210/endo-118-5-1945. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Biden T. J., Farese R. V. Secretin induces rapid increases in inositol trisphosphate, cytosolic Ca2+ and diacylglycerol as well as cyclic AMP in rat pancreatic acini. Biochem J. 1986 Oct 15;239(2):257–261. doi: 10.1042/bj2390257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Verrier B., Fayet G., Lissitzky S. Thyrotropin-binding properties of isolated thyroid cells and their purified plasma membranes. Relation of thyrotropin-specific binding to adenylate-cyclase activation. Eur J Biochem. 1974 Mar 1;42(2):355–365. doi: 10.1111/j.1432-1033.1974.tb03347.x. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Zor U., Lowe I. P., Bloom G., Field J. B. The role of calcium (Ca++) in TSH and dibutyryl 3'5' cyclic AMP stimulation of thyroid glucose oxidation and phospholipid synthesis. Biochem Biophys Res Commun. 1968 Nov 25;33(4):649–658. doi: 10.1016/0006-291x(68)90345-8. [DOI] [PubMed] [Google Scholar]


