Abstract
Nonhuman primate model systems of autologous CD34+ cell transplant are the most effective means to assess the safety and capabilities of lentivirus vectors. Toward this end, we tested the efficiency of marking, gene expression, and transplant of bone marrow and peripheral blood CD34+ cells using a self-inactivating lentivirus vector (CS-Rh-MLV-E) bearing an internal murine leukemia virus long terminal repeat derived from a murine retrovirus adapted to replicate in rhesus macaques. In vitro cytokine stimulation was not required to achieve efficient transduction of CD34+ cells resulting in marking and gene expression of the reporter gene encoding enhanced green fluorescent protein (EGFP) following transplant of the CD34+ cells. Monkeys transplanted with mobilized peripheral blood CD34+ cells resulted in EGFP expression in 1 to 10% of multilineage peripheral blood cells, including red blood cells and platelets, stable for 15 months to date. The relative level of gene expression utilizing this vector is 2- to 10-fold greater than that utilizing a non-self-inactivating lentivirus vector bearing the cytomegalovirus immediate-early promoter. In contrast, in animals transplanted with autologous bone marrow CD34+ cells, multilineage EGFP expression was evident initially but diminished over time. We further tested our lentivirus vector system by demonstrating gene transfer of the human common gamma-chain cytokine receptor gene (γc), deficient in X-linked SCID patients and recently successfully used to treat disease. Marking was 0.42 and .001 HIV-1 vector DNA copy per 100 cells in two animals. To date, all EGFP- and γc-transplanted animals are healthy. This system may prove useful for expression of therapeutic genes in human hematopoietic cells.
Lentivirus vectors based on the human immunodeficiency virus (HIV) genome have been proposed as potential vectors for human hematopoietic progenitor cell gene transfer (3, 6, 12, 14, 30, 38, 43, 47). These vectors have a number of advantages over murine retrovirus vectors, in particular the ability to transduce nondividing cells (32), provided they reside or progress through at least the G1b state of the cell cycle (24). Other vectors based on murine retrovirus genomes are unable to establish infection except when cells progress through mitosis (27, 29, 39). The other advantage of lentivirus vectors is that they have evolved to replicate efficiently in human cells. Thus, lentivirus vectors should in theory provide effective transduction of hematopoietic progenitor cells and maintain high levels of gene expression in differentiated cells. However, these potential advantages and their origin also emphasize the need to adequately assess the properties of lentivirus vectors prior to use in humans. Nonhuman primate models represent the ideal model system to test these vectors in regard to efficacy and safety. This rhesus macaque model system of autologous transplant of CD34+ cells has been used effectively to model human hematopoietic progenitor cell human gene therapy (5, 9, 11, 13, 17, 18, 20, 23, 40, 42, 46, 49). We have previously shown that lentivirus vectors can be used for marking and gene expression following transplant of rhesus macaque CD34+ cells, utilizing mobilized peripheral blood (PB) CD34+ cells (3). Thus, this model system is ideal for evaluation of the efficiency of marking and the efficiency and maintenance of gene expression and for initial safety testing regarding introduction of potential human therapeutic genes.
Here we report on the use of a lentivirus vector that combines the best features of murine retrovirus (murine leukemia virus [MLV]) and HIV type 1 (HIV-1) vectors (25). This vector gives long-term expression in rhesus macaque hematopoietic cells following transplant of transduced mobilized CD34+ PB cells in the absence of in vitro cytokine stimulation. The use of this vector demonstrates that marking is more efficient in mobilized PB cells than in bone marrow (BM) cells. Finally, the vector was used to express the human common gamma-chain cytokine receptor gene (γc) in lymphocytes of rhesus macaques.
MATERIALS AND METHODS
HIV-1 vector construction and production.
Construction of the HIV-1-based vector, pCS-RhMLV-E, was described previously (25). To construct a lentivirus vector carrying the human common γc, (pCS-RhMLV-huγc), pCS-RhMLV-E was first digested with AgeI and XhoI to remove the enhanced green fluorescent protein (EGFP) cDNA and then blunt ended by Klenow fragment. The resulting vector fragment was ligated to the γc cDNA fragment that was isolated from plasmid SRαG1 (45) by XbaI digestion followed by Klenow fragment blunt ending. The vesicular stomatitis virus G protein expression plasmid (pHCMV-G) and the packaging plasmid for HIV-1-based vectors (pCMVR8.2DVPR) were described previously (3). All virus stocks were prepared by calcium phosphate-mediated, three-plasmid transfection of 293T cells (American Type Culture Collection, Manassas, Va.) as described previously (2). In brief, 293T cells (20 × 106), cultured in Dulbecco's modified Eagle medium with 10% calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), were transfected with 5 μg of pHCMV-G, 12.5 μg of pCMVR8.2DVPR, and 12.5 μg of an HIV-1-based vector (pCS-RhMLV-E or pCS-RhMLV-huγc). Virus supernatant was collected on days 2, 3, and 4 posttransfection, filtered through a 0.22-μm-pore-size filter, and concentrated 100-fold by ultracentrifugation. Virus stocks were titrated by infecting 293T cells or HeLa cells (105) with various dilutions of the virus stock and analyzed for EGFP or γc expression by flow cytometry on day 3 postinfection. The titers of vectors were routinely 108 infectious units/ml.
Rhesus leukapheresis procedure and reinfusion of transduced cells in rhesus macaques.
Young adult rhesus macaques (Macaca mulatta) that were serologically negative for simian T-cell lymphotropic virus, simian immunodeficiency virus, simian AIDS-related type D virus, and herpes B virus were used. They were quarantined and housed in accordance with federal guidelines (34) and the policies set by the Veterinary Research Program of the National Institutes of Health. The protocols were evaluated and approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. Four rhesus macaques were injected subcutaneously with a combination of granulocyte colony-stimulating factor (G-CSF; 10 μg/kg of body weight/day) and stem cell factor (SCF; 200 μg/kg/day) (both kindly provided by Amgen, Inc., Thousand Oaks, Calif.) 4 days before the cell harvest (PB or BM). Mobilized (cytokine-stimulated) leukapheresis cell product of the PB from two rhesus macaques (95E132 and 96E035) was harvested by a CS3000 Plus blood cell separator (Baxter Healthcare, Fenwal Division, Deerfield, III.), using a single small-volume chamber and other modifications made to the fluid path of the CS3000 Plus blood cell separator (10). This device allowed leukapheresis procedures to be performed on rhesus macaques weighing less than 5 kg. BM cells were surgically harvested from the femurs and iliac crests of two rhesus macaques (96E041 and 95E131) under anesthesia. After harvest, the PB mononuclear cells (PBMN) and BM mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia) density centrifugation, followed by immunoselection of CD34+ cells. All four animals received a total dose of 10 Gy of total-body irradiation over 2 consecutive days prior to reinfusion of the transduced CD34+ cells. PB was collected on the designated days and evaluated by PCR analysis or flow cytometry for EGFP DNA or expression, respectively.
Immunoselection of nonhuman primate CD34+ cells.
PB- and BM-derived CD34+ cells were isolated according to the manufacturer's instructions by magnetic selection using a biotinylated CD34+ antibody (clone 12.8; CellPro, Inc., Bothell, Wash.), streptavidin MicroBeads, and MACS separation columns (Miltenyi Biotec, Inc., Aubum, Calif.). Purity following the immunoselection procedure was routinely >95%, as assessed by surface staining of allophycocyanin-conjugated anti-CD34 monoclonal antibody (clone 563; a kind gift from Gustav Gaudernack, Institution of Transplantation Immunology, Rikshospitalet, The National Hospital, Oslo, Norway, with allophycocyanin conjugation performed by Molecular Probes, Inc., Eugene, Oreg.) and analysis in an ELITE flow cytometer (Beckman Coulter Corp., Miami, Fla.).
In vitro lentivirus vector transduction.
Immunoselected PB and BM CD34+ cells were transduced as described previously (3). In brief, the cells were cultured on RetroNectin (BioWhittaker, Walkersville, Md.)-coated, non-tissue culture-treated six-well plates (Becton Dickinson Labware, Franklin Lakes, N.J.) and protamine sulfate (8 μg/ml) according to the manufacturer's instructions. They were transduced with the vesicular stomatitis virus G protein-pseudotyped HIV-1 vector (CS-RhMLV-E or CSRhMLV-huγc) for 2 h twice a day for 2 days at a multiplicity of infection (MOI) of approximately 5. The transduced autologous CD34+ cells were reinfused into the irradiated animals for the evaluation of gene expression and of multilineage and long-term marking in vivo. Rhesus macaque PBMN were isolated from mock-infected rhesus macaque PB by Ficoll-Hypaque density separation. They were activated by anti-monkey CD3 (1 mg/ml; BioSource International, Camarillo, Calif.) and anti-human CD28 (1 μg/ml; catalog no. P42235M; Biodesign International, Kennebunk, Main) antibodies and human interleukin-2 (IL-2; 10 U/ml; Amgen) for 2 days as described previously (3). To test the expression of human γc of the vector CS-RhMLV-huγc, HeLa cells (5 × 104) and activated rhesus PBMN (5 × 105) were infected with CS-RhMLV-huγc vector at MOIs 100 and 10 for 2 h at 37°C, respectively. The transduced cells were washed with medium and cultured for 3 days before flow cytometric analysis.
Flow cytometric analysis of rhesus macaque PB hematopoietic cells.
Rhesus macaque PB was obtained from transplanted macaques with lentivirus vector-transduced PB or BM CD34+ cells at various time points postreconstitution. The obtained PB was diluted 100-fold with phosphate-buffered saline (PBS) and analyzed for EGFP expression in red blood cells (RBC) and platelets by flow cytometry. To analyze for EGFP expression in granulocyte, monocyte, and lymphocyte populations, the obtained PB was first incubated with red cell lysis buffer 150 mM (ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM EDTA [pH 7.4]) at 4°C to achieve complete RBC lysis prior to analysis. Each cell populations were gated according to size (forward scatter plot) and granularity (side scatter plot). The cells were analyzed in a FACScan flow cytometer with the CellQuest software (Becton Dickinson). Fifty thousand events were acquired for analysis.
To analyze surface expression of human γc, transduced cells (5 × 105) were first incubated with 50 μl of human AB serum (Omega Scientific, Tarzana, Calif.), 2 μl of Fc Block (PharMingen), and 5 μl of rat immunoglobulin G2b antibodies (Coulter) for 15 min at room temperature to block nonspecific binding. The cells were further incubated on ice for 5 min before the addition of 50 μl of phycoerythrin (PE)-conjugated anti-human γc monoclonal antibodies (diluted to 0.004 mg/ml; Tugh4; catalog no. 351945B; PharMingen). The cells were incubated on ice for 20 min, washed with PBS–1% fetal calf serum, and then resuspended in 7AAD buffer (7AAD [1 μg/ml] in PBS; Calbiochem, San Diego, Calif.) for 30 min on ice before analysis. Dead cells were excluded by gating on the 7AAD-negative population by flow cytometric analysis. Cells that were stained with rat immunoglobulin G2b monoclonal antibodies conjugated with PE (Coulter) were used as the isotype control.
Quantitative PCR assay.
Each PCR amplification was performed as described elsewhere (50). In brief, to detect HIV-1 vector sequences, one of the oligonucleotide primers for each pair used was end labeled with 32P, and 25 ng was included in the reaction (usually 5 × 106 to 1 × 107 cpm). The second oligonucleotide primer was not labeled, and 50 ng was incorporated into each reaction. Each reaction mixture contained 0.25 mM each of the four deoxynucleoside triphosphates, 50 mM NaCl, 25 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 100 μg of bovine serum albumin per ml, and 1.25 U of Taq DNA polymerase (Promega, Madison, Wis.). The reaction mixture was overlaid with 25 μl of mineral oil and then subjected to 25 cycles of denaturation for 1 min at 94°C and polymerization for 2 min at 65°C. The reaction was performed on a Perkin-Elmer thermocycler. Amplified products resulting from the PCR were analyzed by electrophoresis on 6% nondenaturing polyacrylamide gels and visualized by direct autoradiography of the dried gels. Quantitative analysis of the amplified products was performed with a Phosphorimager (Molecular Dynamics, Sunnyvale, Calif.), and data were analyzed with the ImageQuaNT program (Molecular Dynamics). The nucleotide sequences of the oligonucleotide primers (M667 and AA55) used for pCS-RhMLV-E DNA detection were derived from the nucleotide sequence of the HIV-1 long terminal repeat (LTR) as previously described (50). A pair of oligonucleotide primers complementary to the first exon of the human β-globin gene (LA1 and LA2) (50) was used in each reaction mixture in PCR analyses to normalize the total amount of rhesus macaque cellular DNA present. During PCR amplification, labeled β-globin-specific oligonucleotides were incorporated into the reaction at 5 × 106 to 1 × 107 cpm.
HIV-1 vector DNA was quantitated during PCR amplifications by analyzing a standard curve of dilution of pHRCMVEGFP plasmid DNA digested with (HpaI), a restriction enzyme which does not cleave the vector sequence. This DNA was diluted in 0.01 μg of rhesus macaque PBMN DNA per ml. The copy number of HIV-1 vector included in the standard curve ranged from 10 to 3,000. Standard curves for rhesus macaque β-globin DNA were obtained by amplification of 0.001 to 0.03 μg of rhesus macaque cellular DNA (10 to 3,000 cell equivalents) from rhesus macaque PBMN.
RESULTS
Transduction of immunoselected mobilized PB- and BM-derived CD34+ cells with the CS-Rh-MLV-E lentivirus vector.
We previously established a nonhuman primate rhesus macaque transplantation model for the evaluation of lentivirus transduction of CD34+ cells in vivo (3). We used an HIV-1 vector (HR'CMVEGFP) (33) bearing an internal cytomegalovirus (CMV) immediate-early promoter to express EGFP as a reporter gene for assessment of marking efficiencies (3). To date, multilineage PB hematopoietic cells in transplanted rhesus macaques expressed EGFP stably for 2 years. However, gene expression from the HR'CMVEGFP vector was low in rhesus macaque hematopoietic cells. We developed a self-inactivating HIV vector, CS-Rh-MLV-E, which bears an LTR (Rh-MLV) derived from the MLV replication-competent retrovirus found in the sera of one rhesus macaque monkey that developed T-cell lymphoma (25). We previously showed that CS-Rh-MLV-E had 5- to 10-fold-higher EGFP expression in human T cells than a CMV immediate-early promoter-based self-inactivating HIV vector (25). We therefore evaluated the CS-Rh-MLV-E vector in rhesus macaques in the transplantation model for gene expression and for multilineage marking, and long-term reconstitution. We compared immunoselected mobilized PB and BM CD34+ cells of the rhesus macaques that were treated with SCF and G-CSF for their efficiency of lentivirus transduction and reconstitution of rhesus macaque hematopoietic system in vivo. Nonhuman primate immunoselected PB and BM CD34+ cells of four animals were transduced with CS-RhMLV-E twice a day for 2 days in the absence of further cytokine stimulation ex vivo and were analyzed for EGFP expression by flow cytometry 12 h postinfection. Table 1 shows that 15 and 20% of the transduced PB CD34+ cells were EGFP positive for animals 96E035 and 95E132, respectively. In comparison, 52 and 53% of the transduced BM CD34+ cells were EGFP positive (animals 96E041 and 95E131, respectively) (Table 1). Consistent with previously published studies in SCID-NOD mice (30) and in vitro (6, 12, 14, 38, 43, 44, 47), lentivirus vector transduction is achieved in the rhesus macaque CD34+ cells of either PB or BM origin without further cytokine stimulation ex vivo.
TABLE 1.
Outcome of G-CSF- and SCF-mobilized PB- and BM-derived immunoselected CD34+ cells and transplantation of rhesus macaques
| Animal (CD34+ cell origin) | No. of CD34+ cells reinfused (106)a | % EGFP in reinfused CD34+ cellsb | Day of white blood cell count >1,000/μl | Day of platelet count >50,000/μl |
|---|---|---|---|---|
| 96E035 (PB) | 9 | 15 | 16 | 42 |
| 95E132 (PB) | 36 | 20 | 8 | 0c |
| 96E041 (BM) | 36 | 52 | 17 | 18 |
| 95E131 (BM) | 10 | 53 | 18 | 32 |
Nonhuman primate immunoselected CD34+ cells of four animals were transduced with CS-RhMLV-E twice a day for 2 days in the absence of further cytokine stimulation ex vivo at an MOI of approximately 5 and reinfused into autologous animals as described in Materials and Methods.
CS-RhMLV-E-transduced CD34+ cells were analyzed for EGFP expression by flow cytometry 12 h postinfection.
Never fell below 50,000.
Transplantation of rhesus macaque with the lentivirus vector-transduced PB CD34+ cells.
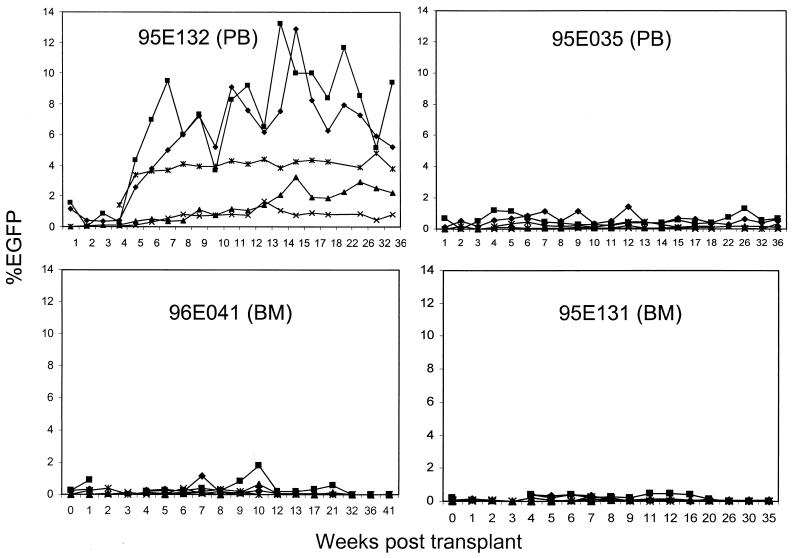
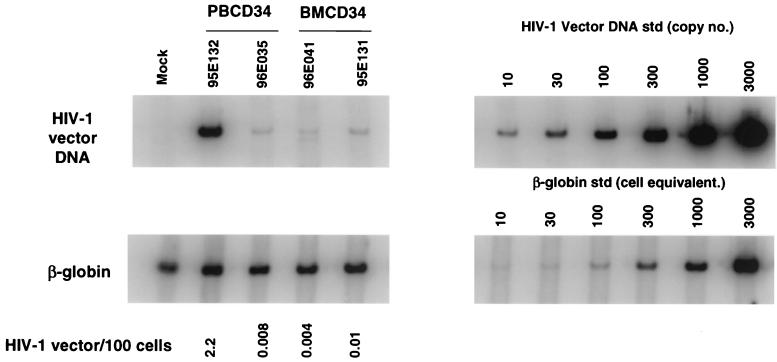
Transduced CD34+ cells were infused into irradiated rhesus macaques to study gene expression, multilineage marking, and long-term reconstitution. Two animals received autologous transplants with transduced PB CD34+ cells (9 × 106 and 36 × 106 cells in 96E035 and 95E132, respectively) (Table 1). All animals received 10 Gy of total-body γ irradiation as a 5-Gy fractionated dose given on 2 consecutive days before transplantation (days −1 and 0, with day 0 being the date of reinfusion). Leukocyte counts recovered to 1,000 cells/μl between days 8 and 18, with platelet counts recovering to greater than 50,000/μl by day 42. One animal, 95E132, never experienced platelet counts below 50,000/μl following transplantation and had leukocyte recovery by day 8. Marking and expression of the vector were monitored in different hematopoietic lineages by flow cytometric analysis for EGFP expression (Fig. 1) and were further confirmed by quantitative DNA PCR (Fig. 2). EGFP expression was detected in PB cells (PBC) from macaques transplanted with autologous PB CD34+ cells beginning at 1 week after transplant. In both animals, EGFP marking was observed in granulocyte, monocyte, lymphocyte, RBC, and platelet populations and has been stable for 65 weeks to date. Animal 96E035 has a lower percentage of EGFP+ cells in all lineages compared to animal 95E132. We also examined the presence of the vector DNA in PBC of animals 95E132 and 96E035 by quantitative DNA PCR analysis at 22 weeks posttransplantation (Fig. 2). The highest EGFP-marked animal (95E132) had highest amount of vector DNA (2.2 copies per 100 cells).
FIG. 1.
Kinetics of EGFP expression following transplantation. The percentage of EGFP-expressing granulocytes (⧫), lymphocytes (▴), monocytes (■), RBC (×), and platelets (✻) for all four animals that received the lentivirus-transduced immunoselected CD34+ cells was determined at various time points as described in Materials and Methods. Animals 95E132 and 96E035 were transplanted with CS-RhMLV-E-transduced PB-derived CD34+ cells; animals 96E041 and 95E131 were transplanted with CS-RhMLV-E-transduced BM-derived CD34+ cells. The percentage of EGFP-expressing cells is shown over a 41-week evaluation period.
FIG. 2.
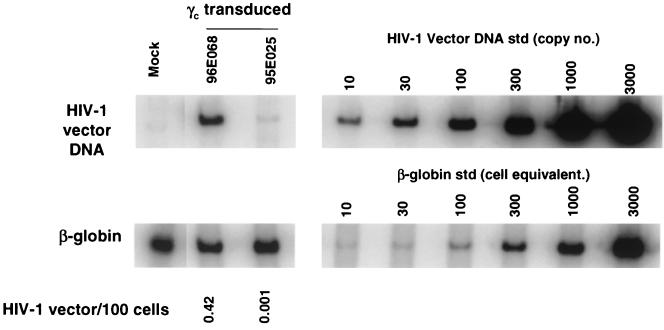
PCR analysis of rhesus macaque cell fractions following transplantation. DNA from rhesus macaque PBC was analyzed for HIV-1 vector transduction by PCR at 22 weeks (95E132 and 96E035), 17 weeks (96E041), and 16 weeks (95E131) after reconstitution of rhesus macaques. HIV-1 vector DNA-specific signal was compared to that of the amplified β-globin DNA signal to determine the number of vector copies per 100 cell (HIV-1 vector DNA copies/100 cells, calculated as number of HIV-1 vector DNA copies/number of cell equivalents × 1/10 × 100). For PCR amplification, 10-fold less DNA was used for β-globin DNA standards (std) in order to obtain quantitative β-globin DNA signals. Quantitative HIV-1 vector DNA and β-globin DNA standards were assayed along with DNA from a nontransduced rhesus (Mock) in parallel; no HIV-1 vector signals were detected from the latter.
EGPF expression from CS-Rh-MLV E was higher than that from HR'CMVEGFP in rhesus macaque PBC.
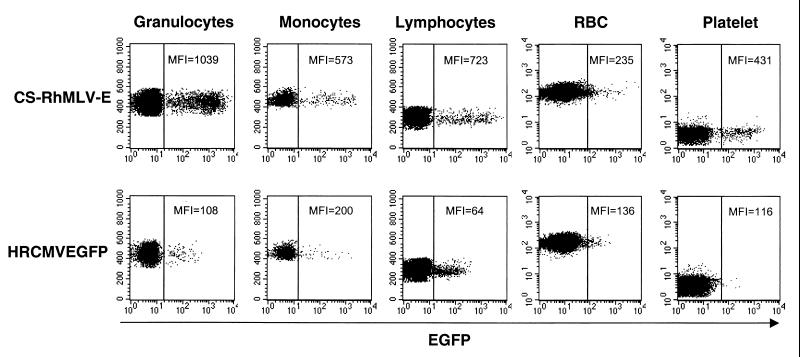
Previously, we showed that the HIV vector bearing the Rh-MLV LTR promoter has 5- to 10-fold-higher EGFP expression in human T lymphocytes than the HR'CMVEGFP vector, bearing the CMV immediate-early promoter in vitro (25). We therefore examined the fluorescence intensity of EGFP expression in multiple lineages of rhesus hematopoietic cells and compared their mean fluorescence intensities (MFI) of EGFP expression to that of the previously described animal (RC505) that was transplanted with CD34+ cells transduced by the CMV promoter-bearing HIV-1 vector (HR'CMVEGFP) (3). The MFI of EGFP expression in the transduced hematopoietic cells of animal RC505 has been stably maintained since week 13 posttransplantation and remains unchanged for 127 weeks to date. We found that the CS-Rh-MLV-E vector consistently gave 2- to 10-fold-greater levels of gene expression than the CMV promoter in granulocyte, monocyte, lymphocyte, RBC, and platelet populations (Fig. 3). To date, this higher level of EGFP expression has been stably maintained for 65 weeks. It should also be noted that similar MFI of EGFP were observed in both animals 95E132 and 96E035, despite a lower percentage of EGFP+ cells in monkey 96E035. Taken together, we confirm that CS-RhMLV-E allows a higher level of EGFP expression in multiple lineages of rhesus macaque hematopoietic cells than HR'CMVEGFP. Since these vectors differ in both self-inactivation and promoter, we cannot differentiate which of these properties is responsible for greater expression, although in vitro, it is attributed primarily to the promoter (25).
FIG. 3.
CS-RhMLV-E vector allows EGFP expression in rhesus macaques. Circulating leukocytes from rhesus macaques transduced with CS-RhMLV-E or HR'CMVEGFP were evaluated for fluorescent intensity of EGFP expression by flow cytometry. Granulocyte, monocyte, lymphocyte, RBC, and platelet populations were identified and gated according to size (forward scatter) and granularity (side scatter) and analyzed for EGFP expression. Representative results from CS-RhMLV-E-transduced rhesus macaque 95E132 (top, 18 weeks posttransplant) and HR'CMVEGFP vector-transduced rhesus macaque RC505 (bottom, 82 weeks posttransplant) are shown. The x axis represents logarithmic fluorescent intensity of EGFP; the y axis represents the forward scatter. Fifty thousand events were acquired for flow cytometric analysis. Samples were analyzed with a FACSCalibur machine (Becton Dickinson) under identical settings.
Long-term marking was not achieved in rhesus macaques transplanted with CS-RhMLV-E-transduced BM CD34+ cells.
Two animals (96E041 and 95E131) received autologous transplants with CS-RhMLV-E-transduced BM CD34+ cells. These animals had received 10 Gy of total-body γ irradiation as a 5-Gy fractionated dose given on 2 consecutive days before transplantation (days −1 and 0, with day 0 being the date of reinfusion). Leukocyte counts recovered to 1,000 cells/μl by day 18, with platelet counts recovering to greater than 50,000/μl by day 32 (Table 1).
The two macaques transplanted with autologous BM CD34+ cells showed a different pattern of reconstitution and marking. No granulocyte and monocyte populations (marked and unmarked) were found at 2 and 3 weeks posttransplantation (Fig. 1). After 3 weeks, some hematopoietic lineages were reconstituted in both animals. Low percentages of EGFP+ cells were initially detected in PBC of both animals and were found to diminish gradually over time. In one animal (96E041), the EGFP marking in all lineages was lost at 32 weeks posttransplantation. In the second animal (95E131), EGFP marking was lost in granulocytes, monocytes, platelets, and RBC at 26 weeks posttransplantation. Marking was observed only in the lymphocyte population at 26 weeks posttransplantation. To date, EGFP marking in the lymphocyte population was has been stable for 61 weeks.
Gene transfer of human γc.
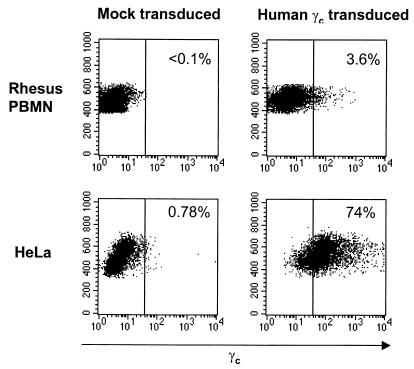
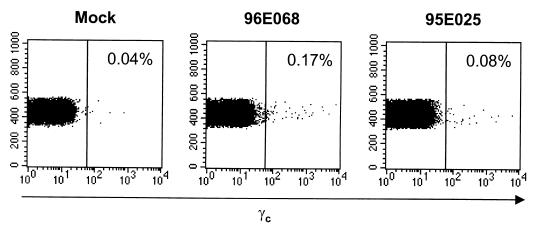
We further tested the potential of our lentiviral vector system by modeling gene transfer of human common γc. Mutations of the common γc have been identified in X-linked SCID patients and have been shown to contribute to the impaired lymphocyte development in these patients (35). Transplantation of the CD34+ cells that were transduced with retrovirus vector carrying the γc cDNA has been shown to restore normal lymphocyte development and functions in the X-linked SCID patients and animal models (7, 28). We inserted human γc cDNA in the place of EGFP cDNA in the CS-Rh-MLV-E vector. Human common γc expression from the vector was confirmed in HeLa cells and rhesus macaque primary PBMC in vitro (Fig. 4). Rhesus macaque immunoselected PB CD34+ cells were transduced twice a day for 2 days with the lentivirus vector bearing human common γc on non-tissue-coated six well plates treated with the recombinant fibronectin fragment CH-296 (RetroNection) without further cytokine stimulation ex vivo. Two animals were transplanted with autologous PB CD34+ cells transduced with the HIV-1 vector bearing human common γc. The gene transfer of γc was determined by quantitative DNA PCR analysis to be 0.42 and 0.001 copies/100 cells (Fig. 5). Cell surface expression of γc was determined by flow cytometric analysis on the rhesus macaque lymphocyte population (Fig. 6). Expression of the γc has been stable for 27 weeks.
FIG. 4.
In vitro transduction of CS-RhMLV-huγc vector. Normal rhesus macaque PBMN (106/ml) were stimulated with immobilized anti-monkey CD3 antibodies, human IL-2, and human CD28 for 2 days as described in Materials and Methods. HeLa cells (5 × 104) or the stimulated PBMN (5 × 105) were infected with CS-RhMLV-huγc vector at MOIs 100 and 10, respectively, as determined by infection of HeLa cells. Three days postinfection, cells were stained with anti-human γc antibodies conjugated with PE as described in Materials and Methods. Ten thousand events were collected for flow cytometric analysis. The percentage of γc-positive lymphocyte populations is indicated in the upper right of each panel. The x axis represents log fluorescent intensity of γc expression; the y axis represents the forward scatter.
FIG. 5.
PCR analysis of human γc-transduced rhesus macaque hematopoietic cells following transplantation. DNA from rhesus macaque PBC was analyzed for the presence of HIV-1 vector DNA by PCR at 7 and 5 weeks after reconstitution of rhesus macaques 96E068 and 95E025, respectively. HIV-1 vector DNA-specific signal was compared to that of the amplified β-globin DNA signal to determine the number of vector copies per 100 cells (HIV-1 vector DNA copies/100 cells, calculated as number of HIV-1 vector DNA copies/number of cell equivalents × 1/10 × 100). For PCR amplification, 10-fold less DNA was used for β-globin DNA standards (std) in order to obtain quantitative β-globin DNA signals. Quantitative HIV-1 vector DNA and β-globin DNA standards were assayed in parallel with DNA from a nontransduced rhesus sample (Mock); no HIV-1 vector signals were detected for the latter.
FIG. 6.
Human γc expression in rhesus macaque lymphocyte population after transplantation. Rhesus macaque PB (5 ml) from one nontransplanted rhesus macaque (Mock) and the two rhesus macaques that were transplanted with CS-RhMLV-huγc-transduced PB CD34+ cells (96E068 and 95E025) was obtained at three separate time points (19, 23, and 27 weeks posttransplant for 96E068; 17, 21, and 25 weeks posttransplant for 95E025). No difference in transduction efficiency was observed at the various time points, and thus representative data from week 23 for 96E068 and week 21 for 95E025 are shown. RBC contamination was eliminated by lysis in red cell lysis buffer before cell surface staining of human γc as described in Materials and Methods. Five hundred thousand cells were stained for human γc as described in Materials and Methods. The lymphocyte population was identified by forward and side scatter plots and analyzed for human γc expression. The percentage of γc-positive lymphocyte populations is indicated in the upper right of each panel. The x axis is log fluorescent intensity of γc expression; the y axis represents the forward scatter.
DISCUSSION
The modeling of gene therapy vectors in nonhuman primate model systems is critical for evaluation of potential efficacy in the clinical setting. In addition, the recent development of lentivirus and retrovirus vectors has led to safety concerns that can best be addressed in nonhuman primate and murine animal model systems including SCID-NOD (4, 8, 15, 16, 26, 30, 31, 37, 41) SCID-hu (1, 3), rhesus macaque (3, 5, 9, 11, 17, 18, 19, 20, 23, 40, 42, 46, 48, 49), and baboon (21, 22). We had previously used a non-self-inactivating lentivirus vector with an internal CMV promoter to demonstrate multilineage marking in rhesus macaques. Those animals were transplanted with ex vivo cytokine-stimulated CD34+ cells transduced with the lentivirus vector. Marking of multiple hematopoietic lineages was achieved in up to 3% of the cells. To date, those animals are stably transduced, nearly 28 months following transplant, and are healthy. Here, we use a self-inactivating lentivirus vector bearing an MLV-related promoter to demonstrate marking in multiple lineages of hematopoietic cells. The CD34+ cells were not stimulated in vitro prior to transduction. The levels of gene expression are significantly higher than that of the non-self-inactivating lentivirus vector utilizing an internal CMV promoter. The level of marking is stable for approximately 15 months to date for those animals transplanted with mobilized PB CD34+ cells. All animals are healthy. Thus, this study represents the first demonstration of the use of lentivirus vectors to transduce non-ex vivo cytokine-stimulated CD34+ cells in a primate.
The results of this study and our previous study (3) indicate considerable variability in the extent of marking between different animals. Such results are consistent with those observed by other investigators using other model systems where the extent of long-term marking with different vectors varies considerably but in most cases is less than 10% (5, 9, 11, 17, 18, 20, 23, 40, 42, 46, 49), with a few exceptions (21, 48, 49). The MOIs of CD34+ cells used here and in our previous study are estimated to be approximately 5. Therefore, it is likely that the extent of marking reflects the relative extent to which progenitor cells are transduced. Given the differences in methodologies for transplant and transduction and use of different animal systems, it is difficult to compare our results with those of other groups. However, we can contrast our results with one other study (by Donohue et al. [11]) and others, where an oncoretrovirus vector expressing EGFP was used for rhesus macaque transplant under similar transplant conditions and in the same facility as that described here, except that the CD34+ cells were stimulated with IL-6, SCF, and fit-3 before transduction. In that study, a transient peak of marking was observed within a few weeks following transplant, most markedly in monocyte and granulocyte lineages, up to as high as 55% marking. The levels of marking in these cells then decreased significantly to less than 0.1%, with long-term maintenance observed only in the lymphocyte subpopulation by several months following transplant. Although transient marking was observed in RBC early following transplant, no significant long-term marking was observed. This kinetics of marking with the oncoretrovirus vector contrasts with that observed with the lentivirus vectors. Both here and in our previous study (3), we did not observe any early transient increase followed by a decline in marking. Rather, the level of EGFP marking rose steadily, peaking within 4 to 5 weeks following transplant and maintained over time. Donohue et al. (11) hypothesized that the early transient marking was the result of infection of committed progenitor cells with less self-renewal capacity. It is possible that the differences observed may reflect the ability of lentivirus vectors to more effectively transduce pluripotent hematopoietic stem cells, thought to be in a more quiescent state.
In contrast to mobilized PB, transduction and transplant of mobilized BM cells did not result in efficient marking. Indeed, in the two animals, marking gradually declined, with loss of marking in most lineages by 26 (95E131) and 32 (96E041) weeks after transplant. There was approximately a 3-week time difference in the rate of reconstitution utilizing PB CD34+ cells compared to BM CD34+ cells. Thus, these results may be due to more efficient reconstitution utilizing PB CD34+ cells or alternatively mobilization of CD34+ target cells from BM to the PB. Our results are consistent with those observed in human clinical studies (36).
The level of marking observed by EGFP expression is consistently higher in the granulocyte lineages followed by monocytes and lymphocytes. This pattern is observed both for the two animals in this study and for four animals in a previous study using the HIV-1-based vector bearing the CMV promoter and therefore appears to be a consistent property of HIV-1-based vectors. The reasons for this are unclear but may relate to greater transduction of progenitors for granulocytic lineages or differential silencing in some lineages. Interestingly, marking was also observed in RBC and platelets. Since these cells do not have genomic DNA, the EGFP must be sufficiently stable to be retained in these cells for detection. The capability to express proteins in RBC and platelets raises a number of potential therapeutic strategies for potential correction of deficiencies in these hematopoietic lineages.
Having developed conditions for transplant and marking, we tested those strategies with a potential human therapeutic gene. Recently, Cavazzana-Calvo et al. demonstrated therapeutic benefit in humans following transplant of γc into X-linked SCID patients, using a murine retrovirus vector (7). We therefore used that gene to model potential human therapeutic gene transfer strategies utilizing the lentivirus gene transfer approach described here. We achieved marking and expression of γc using the lentivirus vector in lymphocytes of the rhesus macaque, utilizing non-cytokine-stimulated CD34+ cells. Although the levels are relatively low, we anticipate that similar to the transplant into X-linked SCID patients with an oncoretrovirus vector (7), under conditions of selective pressure, the γc transduced cells would be expanded. Thus, these studies in nonhuman primate animals provide model strategies and the basis for the future application of lentivirus vectors to potentially treat human diseases.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Betty Poon for critical review of the manuscript, Kazuo Sugamura, Nato Ishi, Hironobu Asao, and Yoshio Koyanagi for providing SRαG1 and valuable conversations, and Liz Duarte for assistance in preparing the manuscript.
This work was supported in part by NIH grants AI36555 and AI39975-01. S.K.P.K. is a Research Fellow of the National Cancer Institute of Canada supported with funds provided by the Terry Fox Run.
REFERENCES
- 1.Akkina R K, Rosenblatt J D, Campbell A G, Chen I S, Zack J A. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood. 1994;84:1393–1398. [PubMed] [Google Scholar]
- 2.An D S, Morizono K, Li Q X, Mao S H, Lu S, Chen I S. An inducible human immunodeficiency virus type 1 (HIV-1) vector which effectively suppresses HIV-1 replication. J Virol. 1999;73:7671–7677. doi: 10.1128/jvi.73.9.7671-7677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An D S, Wersto R P, Agricola B, Metzger M, Lu S, Amado R G, Chen I S Y, Donahue R E. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barquinero J, Segovia J C, Ramirez M, Limon A, Guenechea G, Puig T, Briones J, Garcia J, Bueren J A. Efficient transduction of human hematopoietic repopulating cells generating stable engraftment of transgene-expressing cells in NOD/SCID mice. Blood. 2000;95:3085–3093. [PubMed] [Google Scholar]
- 5.Bodine D M, Moritz T, Donahue R E, Luskey B D, Kessler S W, Martin D I, Orkin S H, Nienhuis A W, Williams D A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993;82:1975–1980. [PubMed] [Google Scholar]
- 6.Case S S, Price M A, Jordan C T, Yu X J, Wang L, Bauer G, Haas D L, Xu D, Stripecke R, Naldini L, Kohn D B, Crooks G M. Stable transduction of quiescent CD34+ CD38− human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Seiz F, Hue C, Certain S, Casanova J L, Bousso P, Deist F L, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 8.Conneally E, Eaves C J, Humphries R K. Efficient retroviral-mediated gene transfer to human cord blood stem cells with in vivo repopulating potential. Blood. 1998;91:3487–3493. [PubMed] [Google Scholar]
- 9.Donahue R E, Kessier S W, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahue R E, Kirby M R, Metzger M E, Agricola B A, Sellers S E, Cullis H M. Peripheral blood CD34+ cells differ from bone marrow CD34+ cells in Thy-1 expression and cell cycle status in nonhuman primates mobilized or not mobilized with granulocyte colony-stimulating factor and/or stem cell factor. Blood. 1996;87:1644–1653. [PubMed] [Google Scholar]
- 11.Donahue R E, Wersto R P, Allay J A, Agricola B A, Metzger M E, Nienhuis A W, Persons D A, Sorrentino B P. High levels of lymphoid expression of enhanced green fluorescent protein in nonhuman primates transplanted with cytokine-mobilized peripheral blood CD34+ cells. Blood. 2000;95:445–452. [PubMed] [Google Scholar]
- 12.Douglas J, Kelly P, Evans J T, Garcia J V. Efficient transduction of human lymphocytes and CD34+ cells via human immunodeficiency virus-based gene transfer vectors. Hum Gene Ther. 1999;10:935–945. doi: 10.1089/10430349950018337. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar C E, Cottler-Fox M, O'Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 14.Evans J T, Kelly P F, O'Neill E, Garcia J V. Human cord blood CD34+ CD38− cell transduction via lentivirus-based gene transfer vectors. Hum Gene Ther. 1999;10:1479–1489. doi: 10.1089/10430349950017815. [DOI] [PubMed] [Google Scholar]
- 15.Follenzi A, Ailles L E, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 16.Guenechea G, Gan O I, Inamitsu T, Dorrell C, Pereira D S, Kelly M, Naldini L, Dick J E. Transduction of human CD34+ CD38− bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 17.Hanazono Y, Brown K E, Handa A, Metzger M E, Heim D, Kurtzman G J, Donahue R E, Dunbar C E. In vivo marking of rhesus monkey lymphocytes by adeno-associated viral vectors: direct comparison with retroviral vectors. Blood. 1999;94:2263–2270. [PubMed] [Google Scholar]
- 18.Heim D A, Hanazono Y, Giri N, Wu T, Childs R, Sellers S E, Muul L, Agricola B A, Metzger M E, Donahue R E, Tisdale J F, Dunbar C E. Introduction of a xenogeneic gene via hematopoietic stem cells leads to specific tolerance in a rhesus monkey model. Mol Ther. 2000;1:533–544. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 19.Hennemann B, Conneally E, Pawliuk R, Leboulch P, Rose-John S, Reid D, Chuo J Y, Humphries R K, Eaves C J. Optimization of retroviral-mediated gene transfer to human NOD/SCID mouse repopulating cord blood cells through a systematic analysis of protocol variables. Exp Hematol. 1999;27:817–825. doi: 10.1016/s0301-472x(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 20.Kaptein L C, Van Beusechem V W, Riviere I, Mulligan R C, Valerio D. Long-term in vivo expression of the MFG-ADA retroviral vector in rhesus monkeys transplanted with transduced bone marrow cells. Hum Gene Ther. 1997;8:1605–1610. doi: 10.1089/hum.1997.8.13-1605. [DOI] [PubMed] [Google Scholar]
- 21.Kiem H P, Andrews R G, Morris J, Peterson L, Heyward S, Allen J M, Rasko J E, Potter J, Miller A D. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 22.Kiem H P, Heyward S, Winkler A, Potter J, Allen J M, Miller A D, Andrews R G. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 23.Kim H J, Tisdale J F, Wu T, Takatoku M, Sellers S E, Zickler P, Metzger M E, Agricola B A, Malley J D, Kato I, Donahue R E, Brown K E, Dunbar C E. Many multipotential gene-marked progenitor or stem cell clones contribute to hematopoiesis in nonhuman primates. Blood. 2000;96:1–8. [PubMed] [Google Scholar]
- 24.Korin Y D, Zack J A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kung S K P, An D S, Chen I S Y. A murine leukemia virus (MuLV) longterminal repeat derived from rhesus macaques in the context of a lentivirus vector and MuLV gag sequence results in high-level gene expression in human T lymphocytes. J Virol. 2000;74:3668–3681. doi: 10.1128/jvi.74.8.3668-3681.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larochelle A, Vormoor J, Hanenberg H, Wang J C, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, Williams D A, Dick J E. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 27.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo M, Bloom M L, Imada K, Berg M, Bollenbacher J M, Bloom E T, Kelsall B L, Leonard W J. Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach. Gene Ther. 1999;94:3027–3036. [PubMed] [Google Scholar]
- 29.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi H, Smith K A, Mosier D E, Verma I M, Torbett B E. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 31.Murray L, Travis M, Luens-Abitorabi K, Olsson K, Plavec I, Forestell S, Hanania E G, Hill B. Addition of the human interferon beta scaffold attachment region to retroviral vector backbones increases the level of in vivo transgene expression among progeny of engrafted human hematopoietic stem cells. Hum Gene Ther. 2000;11:2039–2050. doi: 10.1089/10430340050143453. [DOI] [PubMed] [Google Scholar]
- 32.Naidini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 33.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11383. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institutes of Health. Guide for the care and use of laboratory animals. Department of Health and Human Services Publication no. NIH 85-23(1985). Washington, D.C.: Government Printing Office; 1985. [Google Scholar]
- 35.Noguchi M, Yi H, Rosenblatt H M, Filipovich A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 36.Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J, Shepherd V, Rowland A, Sirohi B, Tait D, Horton C, Long S, Singhal S. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355:1231–1237. doi: 10.1016/S0140-6736(00)02090-0. [DOI] [PubMed] [Google Scholar]
- 37.Quan S, Seiter K, Feldman E, Yang L, Argani I, Farley T J, Abraham N G, Ahmed T. Human CD34+ hematopoietic cells transduced by retrovirus-mediated interferon alpha gene maintains regeneration capacity and engraftment in NOD/SCID mice. Exp Hematol. 1999;27:1511–1518. doi: 10.1016/s0301-472x(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 38.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roe T, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenzweig M, MacVittie T J, Harper D, Hempel D, Glickman R L, Johnson R P, Farese A M, Whiting-Theobald N, Linton G F, Yamasaki G, Jordan C T, Malech H L. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 41.Schiedlmeier B, Kuhlcke K, Eckert H G, Baum C, Zeller W J, Fruehauf S. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood. 2000;95:1237–1248. [PubMed] [Google Scholar]
- 42.Sellers S E, Tisdale J F, Bodine D M, Williams D A, Karlsson S, Meztger M, Donahue R E, Dunbar C E. No discrepancy between in vivo gene marking efficiency assessed in peripheral blood populations compared with bone marrow progenitors or CD34+ cells. Hum Gene Ther. 1999;10:633–640. doi: 10.1089/10430349950018706. [DOI] [PubMed] [Google Scholar]
- 43.Sutton R E, Reitsma M J, Uchida N, Brown P O. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J Virol. 1999;73:3649–3660. doi: 10.1128/jvi.73.5.3649-3660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton R E, Wu H T, Rigg R, Bohnlein E, Brown P O. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 46.Tisdale J F, Hanazono Y, Sellers S E, Agricola B A, Metzger M E, Donahue R E, Dunbar C E. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 47.Uchida N, Sutton R E, Friera A M, He D, Reitsma M J, Chang W C, Veres G, Scollay R, Weissman I L. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu T, Kim H J, Sellers S E, Meade K E, Agricola B A, Metzger M E, Kato I, Donahue R E, Dunbar C E, Tisdale J F. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol Ther. 2000;1:285–293. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- 49.Xu L C, Karlsson S, Byrne E R, Kluepfel-Stahl S, Kessler S W, Agricola B A, Sellers S, Kirby M, Dunbar C E, Brady R O. Long-term in vivo expression of the human glucocerebrosidase gene in nonhuman primates after CD34+ hematopoietic cell transduction with cell- free retroviral vector preparations. Proc Natl Acad Sci USA. 1995;92:4372–4376. doi: 10.1073/pnas.92.10.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]