Abstract
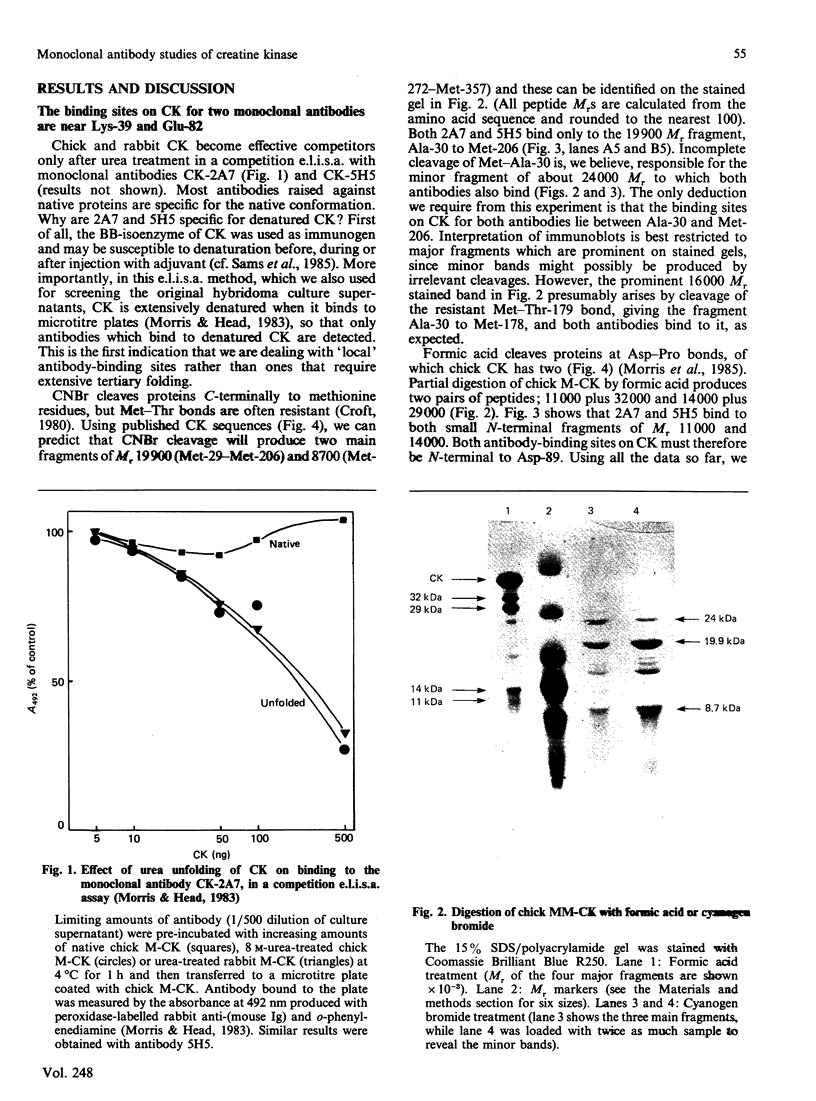
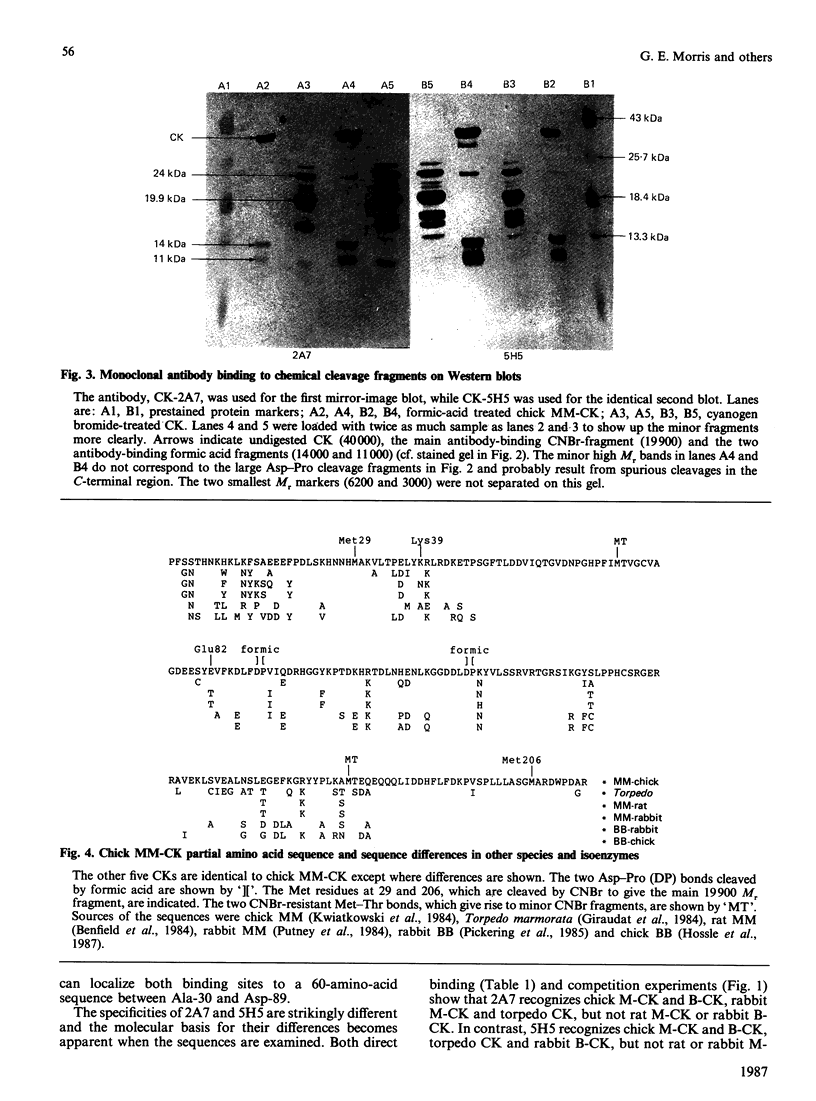
(1) The binding sites of two monoclonal antibodies, CK-2A7 and CK-5H5, have been located to a 60-amino-acid sequence in the N-terminal region of creatine kinase (CK) by the use of chemical cleavage with formic acid (which cleaves proteins at Asp-Pro bonds) and cyanogen bromide (which cleaves at Met residues). (2) A simple method for preparing chemically-cleaved fragments of proteins for electrophoresis and Western blotting is described. (3) Binding studies with CK preparations from different animal species show that single amino acid changes at residues 39 or 82 prevent binding of CK-2A7 and CK-5H5 respectively. We suggest that Lys-39 and Glu-82 form parts of the binding sites on CK for the two monoclonal antibodies. The two sites lie in variable regions at each end of a highly-conserved sequence (residues 46 to 79) and are inaccessible to antibody in the native enzyme. (4) One of the antibodies, CK-2A7, inhibits the refolding of CK to native enzyme after denaturation by urea.
Full text
PDF

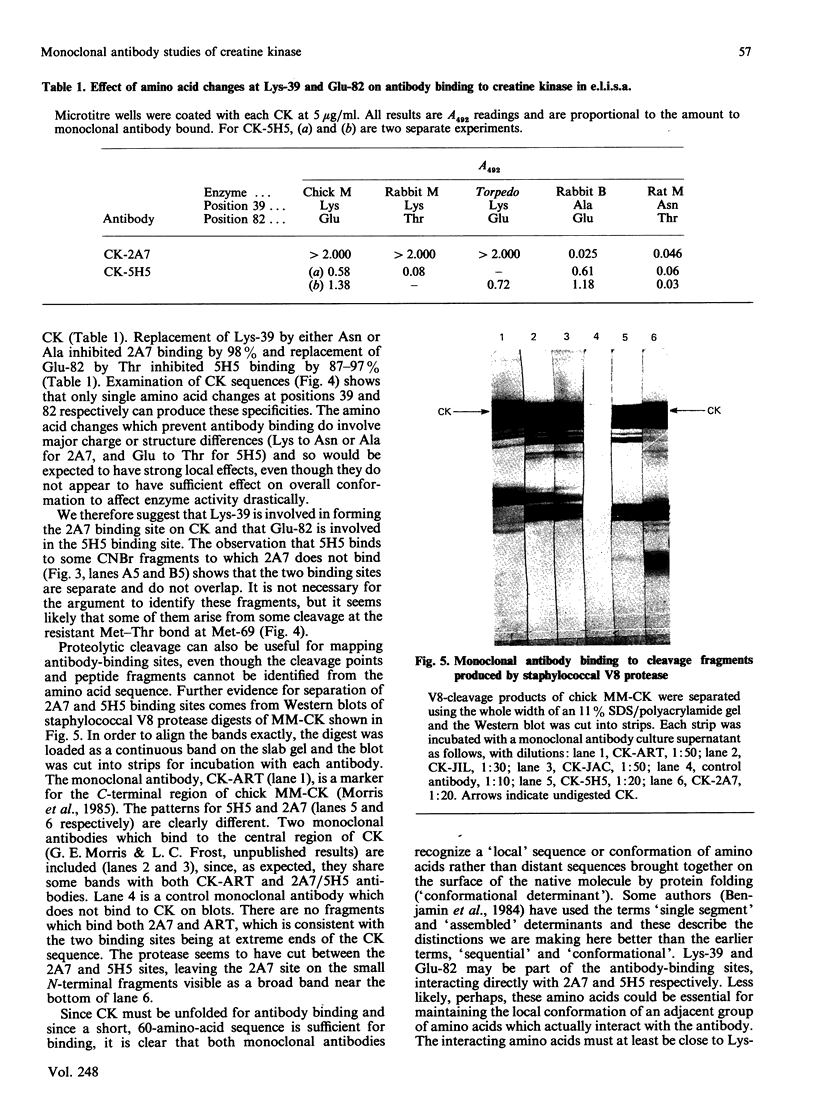
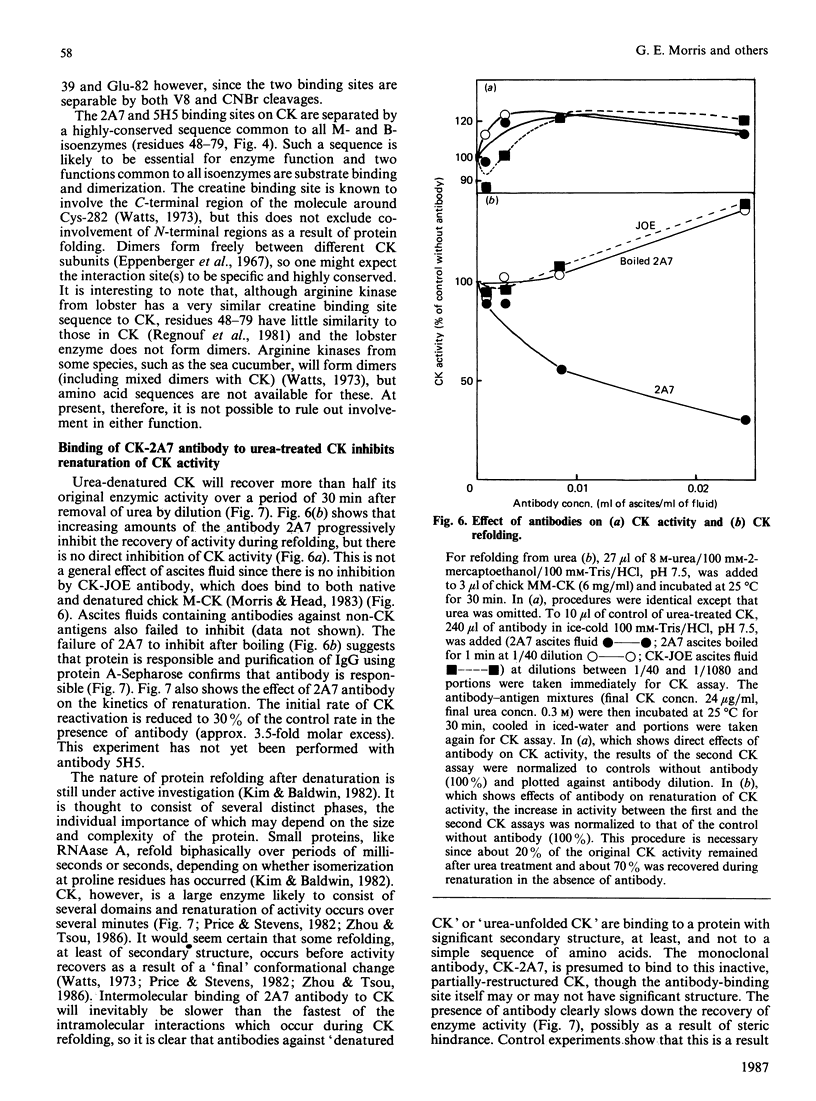
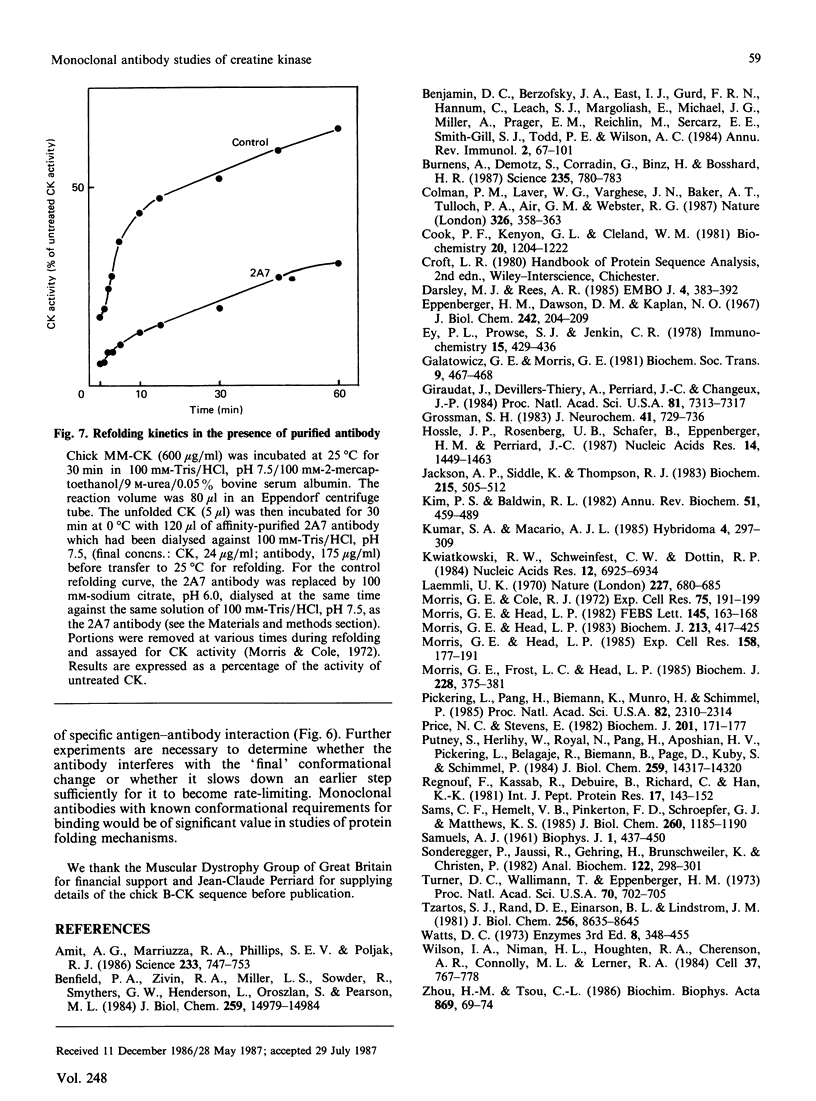
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Benfield P. A., Zivin R. A., Miller L. S., Sowder R., Smythers G. W., Henderson L., Oroszlan S., Pearson M. L. Isolation and sequence analysis of cDNA clones coding for rat skeletal muscle creatine kinase. J Biol Chem. 1984 Dec 10;259(23):14979–14984. [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Burnens A., Demotz S., Corradin G., Binz H., Bosshard H. R. Epitope mapping by chemical modification of free and antibody-bound protein antigen. Science. 1987 Feb 13;235(4790):780–783. doi: 10.1126/science.2433768. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Kenyon G. L., Cleland W. W. Use of pH studies to elucidate the catalytic mechanism of rabbit muscle creatine kinase. Biochemistry. 1981 Mar 3;20(5):1204–1210. doi: 10.1021/bi00508a023. [DOI] [PubMed] [Google Scholar]
- Darsley M. J., Rees A. R. Three distinct epitopes within the loop region of hen egg lysozyme defined with monoclonal antibodies. EMBO J. 1985 Feb;4(2):383–392. doi: 10.1002/j.1460-2075.1985.tb03640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Perriard J. C., Changeux J. P. Complete nucleotide sequence of Torpedo marmorata mRNA coding for the 43,000-dalton nu 2 protein: muscle-specific creatine kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7313–7317. doi: 10.1073/pnas.81.23.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. H. Interaction of creatine kinase from monkey brain with substrate: analysis of kinetics and fluorescence polarization. J Neurochem. 1983 Sep;41(3):729–736. doi: 10.1111/j.1471-4159.1983.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Hossle J. P., Rosenberg U. B., Schäfer B., Eppenberger H. M., Perriard J. C. The primary structure of chicken B-creatine kinase and evidence for heterogeneity of its mRNA. Nucleic Acids Res. 1986 Feb 11;14(3):1449–1463. doi: 10.1093/nar/14.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Siddle K., Thompson R. J. A monoclonal antibody to human brain-type creatine kinase. Increased avidity with mercaptans. Biochem J. 1983 Dec 1;215(3):505–512. doi: 10.1042/bj2150505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Kumar S. A., Macario A. J. Comparative analysis of rat uterine estrogen-induced creatine kinase with monoclonal antibodies. Hybridoma. 1985 Winter;4(4):297–309. doi: 10.1089/hyb.1985.4.297. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski R. W., Schweinfest C. W., Dottin R. P. Molecular cloning and the complete nucleotide sequence of the creatine kinase-M cDNA from chicken. Nucleic Acids Res. 1984 Sep 25;12(18):6925–6934. doi: 10.1093/nar/12.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Cell fusion and differentiation in cultured chick muscle cells. Exp Cell Res. 1972 Nov;75(1):191–199. doi: 10.1016/0014-4827(72)90536-8. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Frost L. C., Head L. P. Monoclonal-antibody studies of creatine kinase. The proteinase K-cleavage site. Biochem J. 1985 Jun 1;228(2):375–381. doi: 10.1042/bj2280375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. E., Head L. P. A monoclonal antibody against the skeletal muscle enzyme, creatine kinase. FEBS Lett. 1982 Aug 16;145(1):163–168. doi: 10.1016/0014-5793(82)81228-3. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Head L. P. Immunoassay of muscle-specific creatine kinase with a monoclonal antibody and application to myogenesis and muscular dystrophy. Biochem J. 1983 Aug 1;213(2):417–425. doi: 10.1042/bj2130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. E., Head L. P. Monoclonal antibodies to intermediate filaments in chick muscle cell cultures. Exp Cell Res. 1985 May;158(1):177–191. doi: 10.1016/0014-4827(85)90442-2. [DOI] [PubMed] [Google Scholar]
- Pickering L., Pang H., Biemann K., Munro H., Schimmel P. Two tissue-specific isozymes of creatine kinase have closely matched amino acid sequences. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2310–2314. doi: 10.1073/pnas.82.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. C., Stevens E. The refolding of denatured rabbit muscle creatine kinase. Search for intermediates in the refolding process and effect of modification at the reactive thiol group on refolding. Biochem J. 1982 Jan 1;201(1):171–177. doi: 10.1042/bj2010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S., Herlihy W., Royal N., Pang H., Aposhian H. V., Pickering L., Belagaje R., Biemann K., Page D., Kuby S. Rabbit muscle creatine phosphokinase. CDNA cloning, primary structure and detection of human homologues. J Biol Chem. 1984 Dec 10;259(23):14317–14320. [PubMed] [Google Scholar]
- Regnouf F., Kassab R., Debuire B., Richard C., Han K. K. Primary structure of lobster-muscle arginine kinase. Amino and carboxyl-terminal structure of the enzyme and complete alignment of the cyanogen-bromide peptides. Int J Pept Protein Res. 1981 Feb;17(2):143–155. doi: 10.1111/j.1399-3011.1981.tb01977.x. [DOI] [PubMed] [Google Scholar]
- SAMUELS A. J. Immunoenzymological evidence suggesting a change in conformation of adenylic acid deaminase and creatine kinase during substrate combination. Biophys J. 1961 Jul;1:437–444. doi: 10.1016/s0006-3495(61)86901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams C. F., Hemelt V. B., Pinkerton F. D., Schroepfer G. J., Jr, Matthews K. S. Exposure of antigenic sites during immunization. Monoclonal antibodies to monomer of lactose repressor protein. J Biol Chem. 1985 Jan 25;260(2):1185–1190. [PubMed] [Google Scholar]
- Sonderegger P., Jaussi R., Gehring H., Brunschweiler K., Christen P. Peptide mapping of protein bands from polyacrylamide gel electrophoresis by chemical cleavage in gel pieces and re-electrophoresis. Anal Biochem. 1982 May 15;122(2):298–301. doi: 10.1016/0003-2697(82)90285-8. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zhou H. M., Tsou C. L. Comparison of activity and conformation changes during refolding of urea-denatured creatine kinase. Biochim Biophys Acta. 1986 Jan 17;869(1):69–74. doi: 10.1016/0167-4838(86)90311-0. [DOI] [PubMed] [Google Scholar]