Abstract
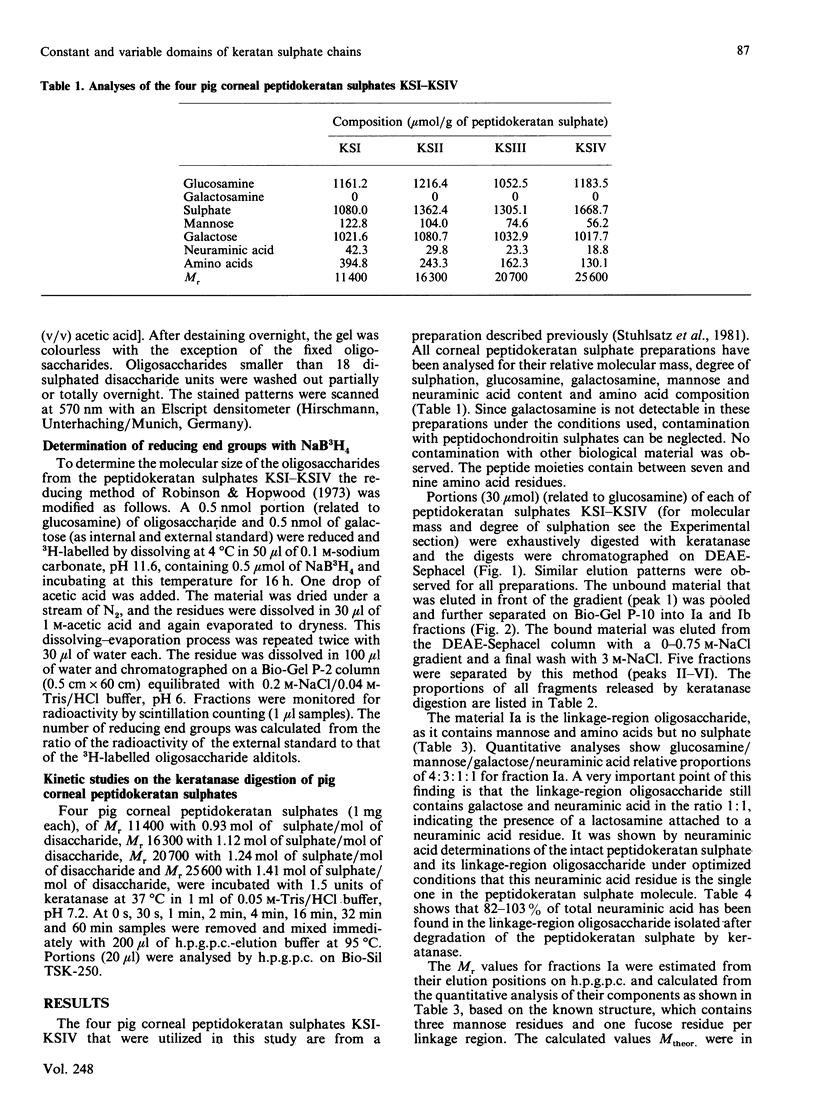
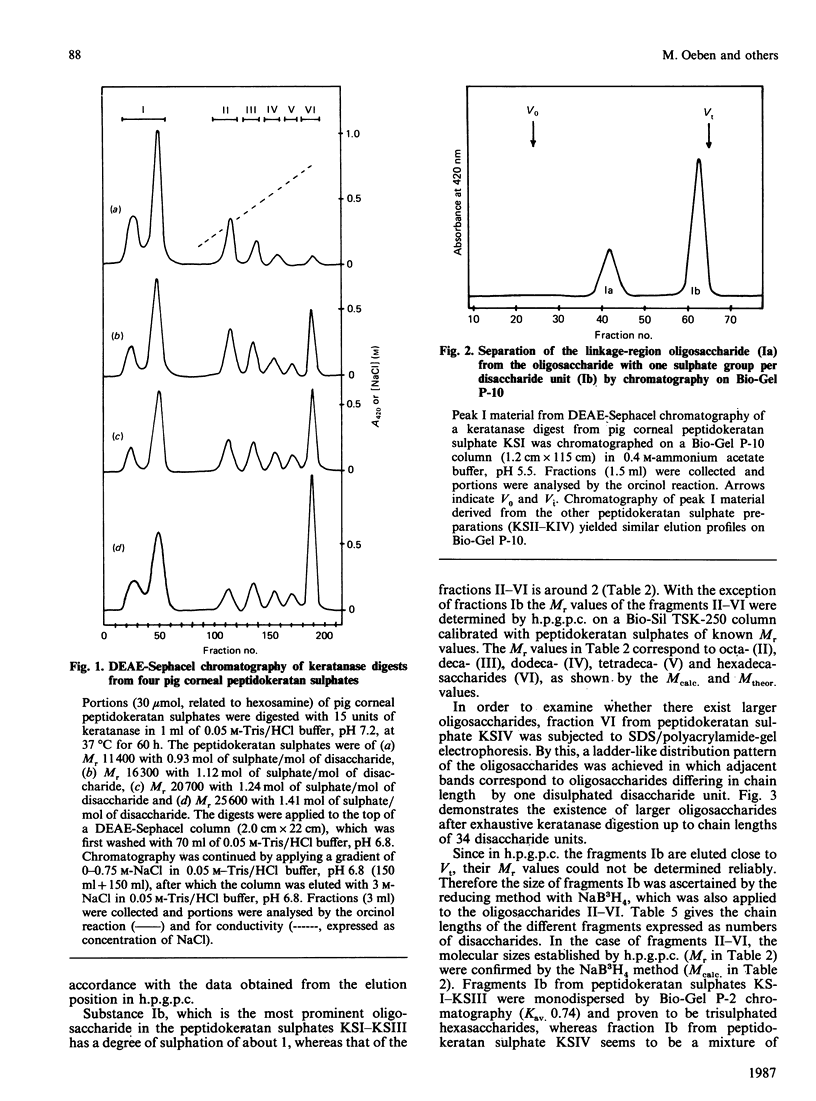
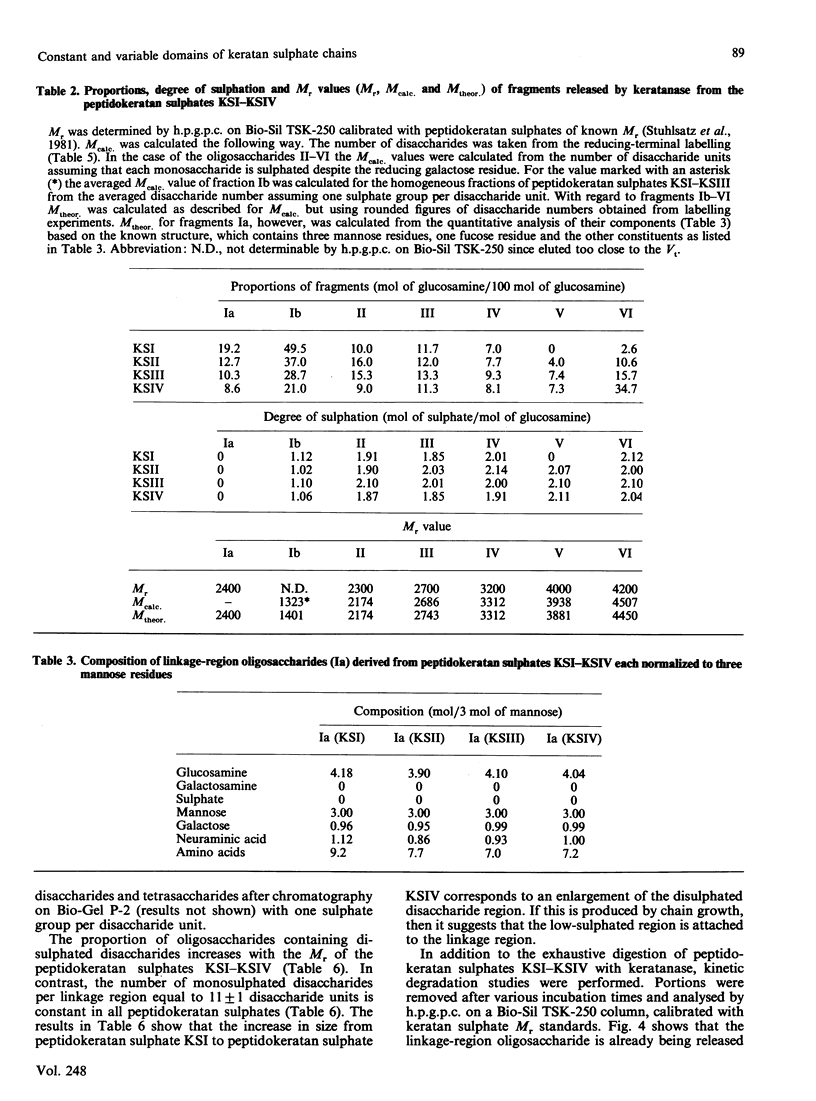
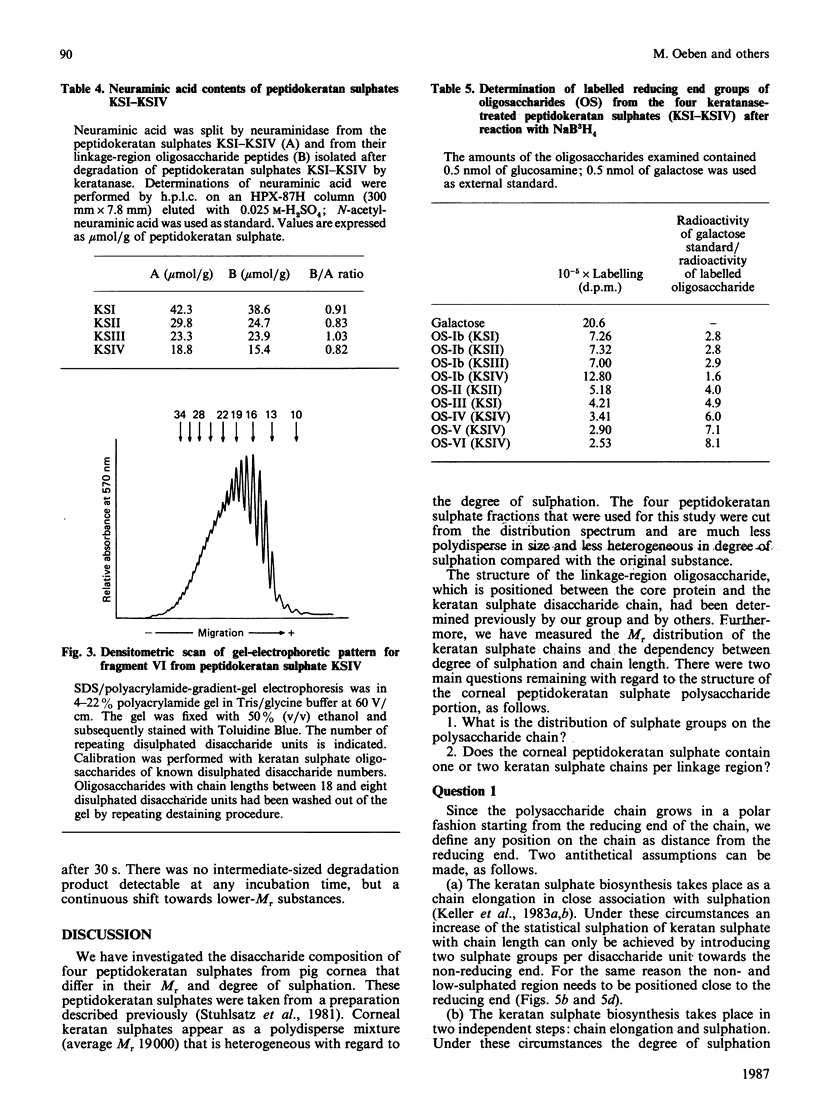
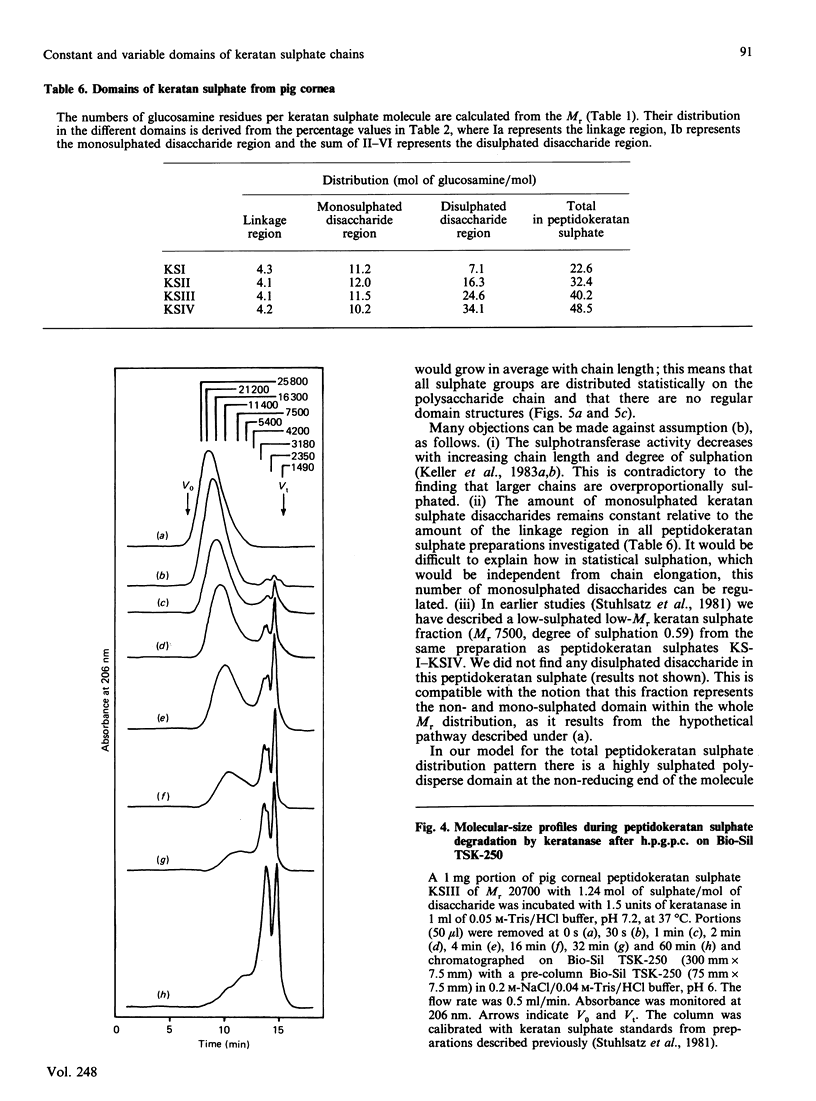
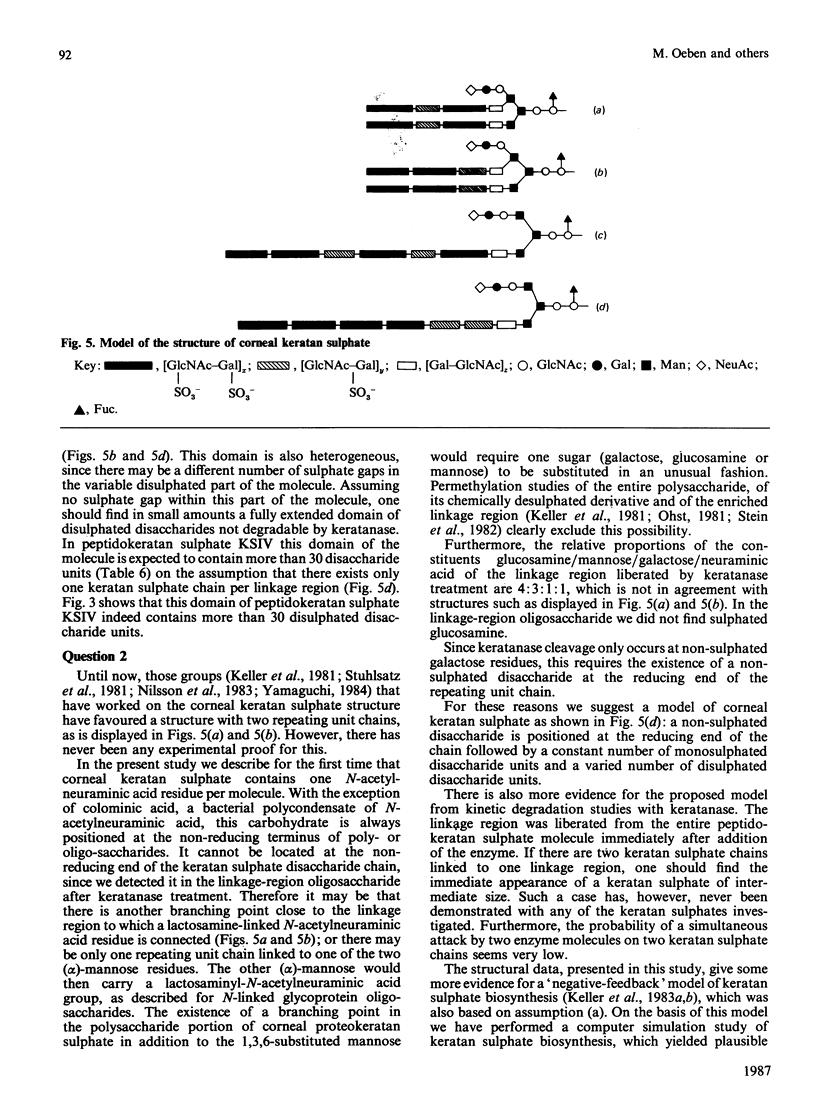
Four peptidokeratan sulphate fractions of different Mr and degree of sulphation were cut from the pig corneal keratan sulphate distribution spectrum. After exhaustive digestion with keratanase, the fragments were separated on DEAE-Sephacel and Bio-Gel P-10 and analysed for their Mr, degree of sulphation and amino sugar and neutral sugar content. It was found that every glycosaminoglycan chain is constructed of a constant domain of non-sulphated and monosulphated disaccharide units and a variable domain of disulphated disaccharide units. Total neuraminic acid of the four peptidokeratan sulphates was recovered from their isolated linkage-region oligosaccharides. In kinetic studies, the four peptidokeratan sulphates were investigated for Mr distribution after various incubation times with keratanase. There was a continuous shift towards lower Mr and no appearance of a distinct intermediate-sized product at any degradation time. The linkage-region oligosaccharide was already being liberated after a very short incubation period. From the results of these kinetic investigations in connection with the results of neuraminic acid analyses it is suggested that there exists only one disaccharide chain per peptidokeratan sulphate molecule. A model of corneal keratan sulphate is postulated. One of the alpha-mannose residues in the linkage region is bound to an oligosaccharide consisting of a lactosamine and a terminal sialic acid. The other alpha-mannose residue is attached to the disaccharide chain. This chain contains one or two non-sulphated disaccharide units at the reducing end, followed by 10-12 monosulphated disaccharide units. The disulphated disaccharide moiety of variable length is positioned at the non-reducing end of the chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brekle A., Mersmann G. The carbohydrate-protein binding region in keratan sulfate from bovine cornea. I. Isolation and partial characterization. Hoppe Seylers Z Physiol Chem. 1980 Jan;361(1):31–39. doi: 10.1515/bchm2.1980.361.1.31. [DOI] [PubMed] [Google Scholar]
- Keller R., Driesch R., Stein T., Momburg M., Stuhlsatz H. W., Greiling H., Franke H. Biosynthesis of proteokeratan sulfate in the bovine cornea. 1) Isolation and characterization of a keratan sulfotransferase and the role of sulfation for the chain termination. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):239–252. doi: 10.1515/bchm2.1983.364.1.239. [DOI] [PubMed] [Google Scholar]
- Keller R., Stein T., Stuhlsatz H. W., Greiling H., Ohst E., Müller E., Scharf H. D. Studies on the characterization of the linkage-region between polysaccharide chain and core protein in bovine corneal proteokeratan sulfate. Hoppe Seylers Z Physiol Chem. 1981 Mar;362(3):327–336. doi: 10.1515/bchm2.1981.362.1.327. [DOI] [PubMed] [Google Scholar]
- Keller R., Stein T., Weber W., Kehrer T., Stuhlsatz H. W., Greiling H., Keyserlingk D. G. Biosynthesis of proteokeratan sulfate in the bovine cornea. 2) Isolation of subcellular membrane fragments from bovine cornea cells with keratan sulfate synthesizing activity. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):253–260. [PubMed] [Google Scholar]
- Nakazawa K., Newsome D. A., Nilsson B., Hascall V. C., Hassell J. R. Purification of keratan sulfate proteoglycan from monkey cornea. J Biol Chem. 1983 May 25;258(10):6051–6055. [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Nilsson B., Nakazawa K., Hassell J. R., Newsome D. A., Hascall V. C. Structure of oligosaccharides and the linkage region between keratan sulfate and the core protein on proteoglycans from monkey cornea. J Biol Chem. 1983 May 25;258(10):6056–6063. [PubMed] [Google Scholar]
- Robinson H. C., Hopwood J. J. The alkaline cleavage and borohydride reduction of cartilage proteoglycan. Biochem J. 1973 Jul;133(3):457–470. doi: 10.1042/bj1330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel K., Bernt E., Schmitz F., Grimmel K. Enzymatische Galaktosebestimmung im Blut und oraler Galaktose-Toleranztest. Klin Wochenschr. 1968 Sep 1;46(17):936–940. doi: 10.1007/BF01747158. [DOI] [PubMed] [Google Scholar]
- Scudder P., Tang P. W., Hounsell E. F., Lawson A. M., Mehmet H., Feizi T. Isolation and characterization of sulphated oligosaccharides released from bovine corneal keratan sulphate by the action of endo-beta-galactosidase. Eur J Biochem. 1986 Jun 2;157(2):365–373. doi: 10.1111/j.1432-1033.1986.tb09678.x. [DOI] [PubMed] [Google Scholar]
- Stein T., Keller R., Stuhlsatz H. W., Greiling H., Ohst E., Müller E., Scharf H. D. Structure of the linkage-region between polysaccharide chain and core protein in bovine corneal proteokeratan sulfate. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):825–833. doi: 10.1515/bchm2.1982.363.2.825. [DOI] [PubMed] [Google Scholar]
- Stuhlsatz H. W., Hirtzel F., Keller R., Cosma S., Greiling H. Studies on the polydispersity and heterogeneity of proteokeratan sulfate from calf and porcine cornea. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):841–852. doi: 10.1515/bchm2.1981.362.2.841. [DOI] [PubMed] [Google Scholar]
- Stuhlsatz H. W., Kisters R., Wollmer A., Greiling H. Zur Struktur von Proteoglykanen aus der Cornea. Hoppe Seylers Z Physiol Chem. 1971 Feb;352(2):289–303. [PubMed] [Google Scholar]
- Yamaguchi H. Studies on the carbohydrate-protein linkage region in bovine corneal keratan sulfate. I. Isolation of linkage-region glycopeptides under mild conditions. J Biochem. 1983 Jul;94(1):207–213. doi: 10.1093/oxfordjournals.jbchem.a134331. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H. Studies on the carbohydrate-protein linkage region in bovine corneal keratan sulfate. II. Structural studies on linkage region-enriched neutral glycopeptides. J Biochem. 1983 Jul;94(1):215–221. doi: 10.1093/oxfordjournals.jbchem.a134332. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H. Studies on the carbohydrate-protein linkage region in bovine corneal keratan sulfate. III. Evidence for a biantennary structure. J Biochem. 1984 Feb;95(2):601–604. doi: 10.1093/oxfordjournals.jbchem.a134647. [DOI] [PubMed] [Google Scholar]
- Ziegler C., Mersmann G. The carbohydrate-protein-binding region in proteokeratan sulfate from bovine cornea is not sulfated. Hoppe Seylers Z Physiol Chem. 1983 Jan;364(1):97–100. [PubMed] [Google Scholar]


