Abstract
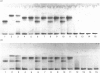
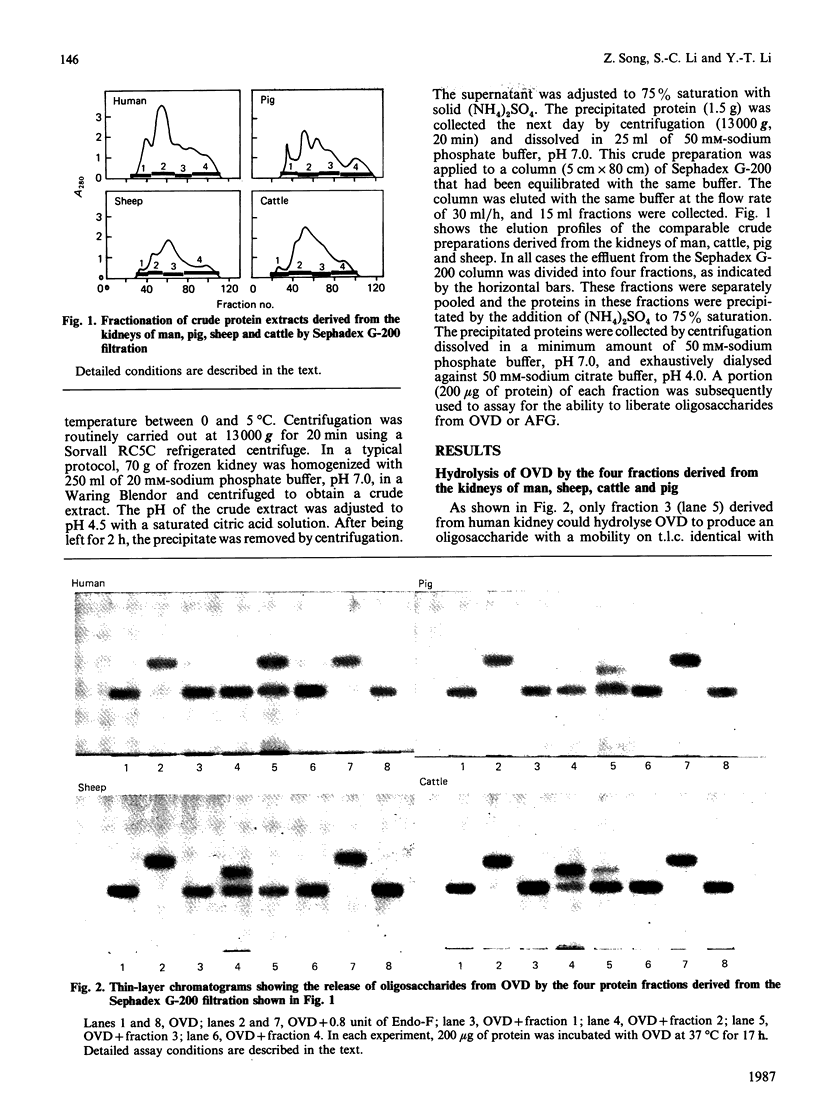
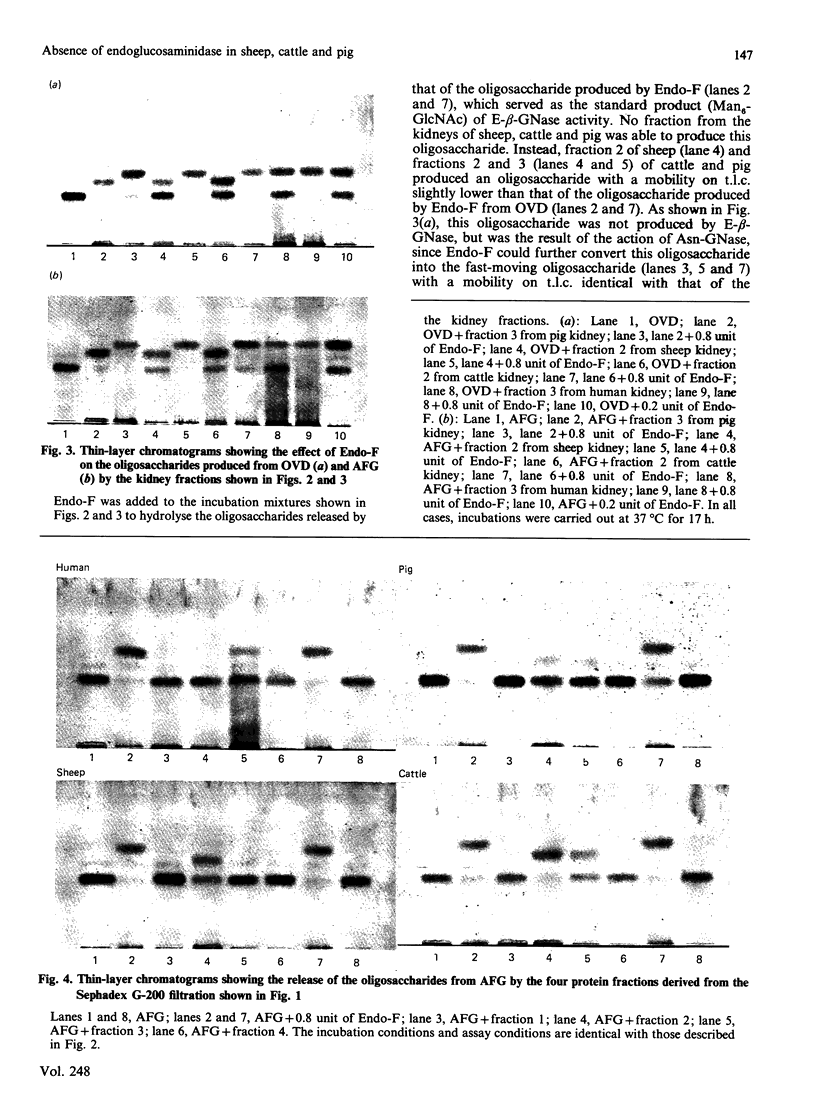
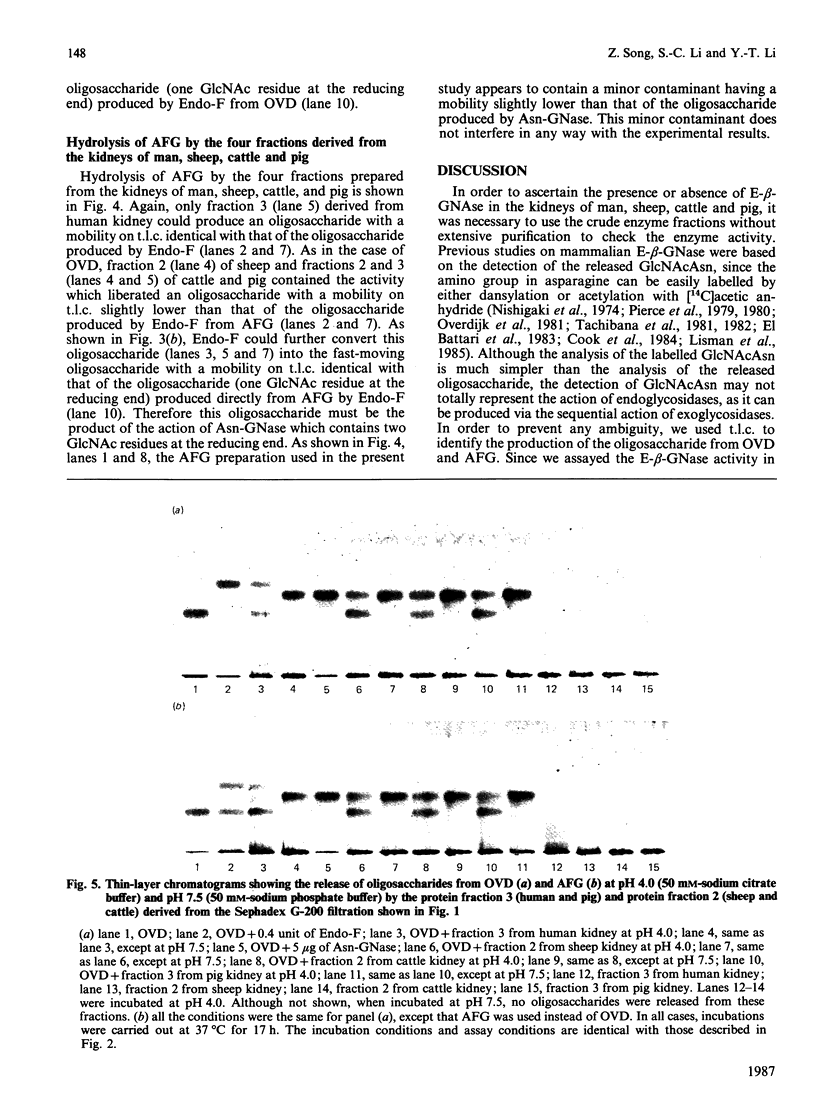
The kidneys of man, sheep, cattle and pig were all found to contain 1-aspartamido-beta-acetylglucosamine amidohydrolase activity. However, among these, only human kidney was found to contain endo-beta-N-acetylglucosaminidase activity. The absence of this enzyme in the kidneys of sheep and cattle explains why the oligosaccharides accumulated in, and excreted by, sheep and cattle afflicted with disorders of glycoprotein catabolism (i.e. alpha-mannosidosis and beta-mannosidosis) contain two N-acetylglucosamine residues at the reducing terminus instead of one, as is the case for human patients afflicted with similar disorders.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D., Blakemore W. F., Jolly R. D., Sidebotham R., Winchester B. The catabolism of mammalian glycoproteins. Comparison of the storage products in bovine, feline and human mannosidosis. Biochem J. 1983 Dec 1;215(3):573–579. doi: 10.1042/bj2150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baussant T., Strecker G., Wieruszeski J. M., Montreuil J., Michalski J. C. Catabolism of glycoprotein glycans. Characterization of a lysosomal endo-N-acetyl-beta-D-glucosaminidase specific for glycans with a terminal chitobiose residue. Eur J Biochem. 1986 Sep 1;159(2):381–385. doi: 10.1111/j.1432-1033.1986.tb09879.x. [DOI] [PubMed] [Google Scholar]
- Cook N. J., Zanetta J. P., Vincendon G. Thin-layer chromatographic assay for endo-beta-N-acetylglucosaminidase activity in rat tissues. J Chromatogr. 1984 Jun 1;292(2):479–484. doi: 10.1016/s0021-9673(01)83632-3. [DOI] [PubMed] [Google Scholar]
- Daniel P. F., Warren C. D., James L. F. Swainsonine-induced oligosaccharide excretion in sheep. Time-dependent changes in the oligosaccharide profile. Biochem J. 1984 Aug 1;221(3):601–607. doi: 10.1042/bj2210601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling P. R., Huxtable C. R., Colegate S. M. Inhibition of lysosomal alpha-mannosidase by swainsonine, an indolizidine alkaloid isolated from Swainsona canescens. Biochem J. 1980 Nov 1;191(2):649–651. doi: 10.1042/bj1910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugal B., Stromme J. Purification and some properties of 1-aspartamido-beta-N-acetylglucosamine amidohydrolase from human liver. Biochem J. 1977 Sep 1;165(3):497–502. doi: 10.1042/bj1650497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock L. W., Jones M. Z., Dawson G. Glycoprotein metabolism in normal and beta-mannosidase-deficient cultured goat skin fibroblasts. Biochem J. 1986 Feb 15;234(1):175–183. doi: 10.1042/bj2340175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. Z., Laine R. A. Caprine oligosaccharide storage disease. Accumulation of beta-mannosyl (1 goes to 4) beta-N-acetylglucosaminyl (1 goes to 4) beta-N-acetylglucosamine in brain. J Biol Chem. 1981 May 25;256(10):5181–5184. [PubMed] [Google Scholar]
- Kuranda M. J., Aronson N. N., Jr A di-N-acetylchitobiase activity is involved in the lysosomal catabolism of asparagine-linked glycoproteins in rat liver. J Biol Chem. 1986 May 5;261(13):5803–5809. [PubMed] [Google Scholar]
- Lisman J. J., van der Wal C. J., Overdijk B. Endo-N-acetyl-beta-D-glucosaminidase activity in rat liver. Studies on substrate specificity, enzyme inhibition, subcellular localization and partial purification. Biochem J. 1985 Jul 15;229(2):379–385. doi: 10.1042/bj2290379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- Nishigaki M., Muramatsu T., Kobata A. Endoglycosidases acting on carbohydrate moieties of glycoproteins: demonstration in mammalian tissue. Biochem Biophys Res Commun. 1974 Jul 24;59(2):638–645. doi: 10.1016/s0006-291x(74)80027-6. [DOI] [PubMed] [Google Scholar]
- Overdijk B., van der Kroef W. M., Lisman J. J., Pierce R. J., Montreuil J., Spik G. Demonstration and partial characterization of endo-N-acetyl-beta-D-glucosaminidase in human tissues. FEBS Lett. 1981 Jun 15;128(2):364–366. doi: 10.1016/0014-5793(81)80118-4. [DOI] [PubMed] [Google Scholar]
- Pierce R. J., Spik G., Montreuil J. Cytosolic location of an endo-N-acetyl-beta-D-glucosaminidase activity in rat liver and kidney. Biochem J. 1979 Jun 15;180(3):673–676. doi: 10.1042/bj1800673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R. J., Spik G., Montreuil J. Demonstration and cytosolic location of an endo-N-acetyl-beta-D-glucosaminidase activity towards an asialo-N-acetyl-lactosaminic-type substrate in rat liver. Biochem J. 1980 Jan 1;185(1):261–264. doi: 10.1042/bj1850261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Tachibana Y., Yamashita K., Kawaguchi M., Arashima S., Kobata A. Digestion of asparagine-linked oligosaccharides by endo-beta-N-acetylglucosaminidase in the human skin fibroblasts obtained from fucosidosis patients. J Biochem. 1981 Nov;90(5):1291–1296. doi: 10.1093/oxfordjournals.jbchem.a133594. [DOI] [PubMed] [Google Scholar]
- Tachibana Y., Yamashita K., Kobata A. Substrate specificity of mammalian endo-beta-N-acetylglucosaminidase: study with the enzyme of rat liver. Arch Biochem Biophys. 1982 Mar;214(1):199–210. doi: 10.1016/0003-9861(82)90023-6. [DOI] [PubMed] [Google Scholar]
- Townsend R. R., Hilliker E., Li Y. T., Laine R. A., Bell W. R., Lee Y. C. Carbohydrate structure of human fibrinogen. Use of 300-MHz 1H-NMR to characterize glycosidase-treated glycopeptides. J Biol Chem. 1982 Aug 25;257(16):9704–9710. [PubMed] [Google Scholar]