Abstract
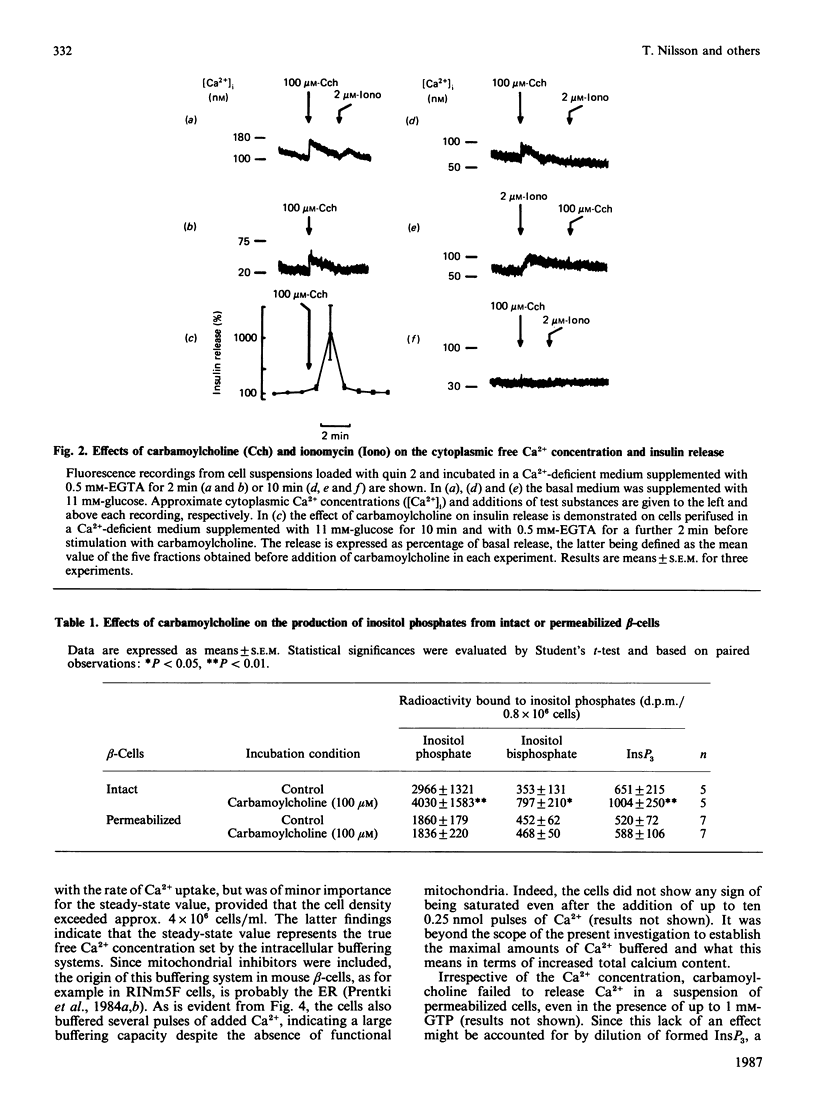
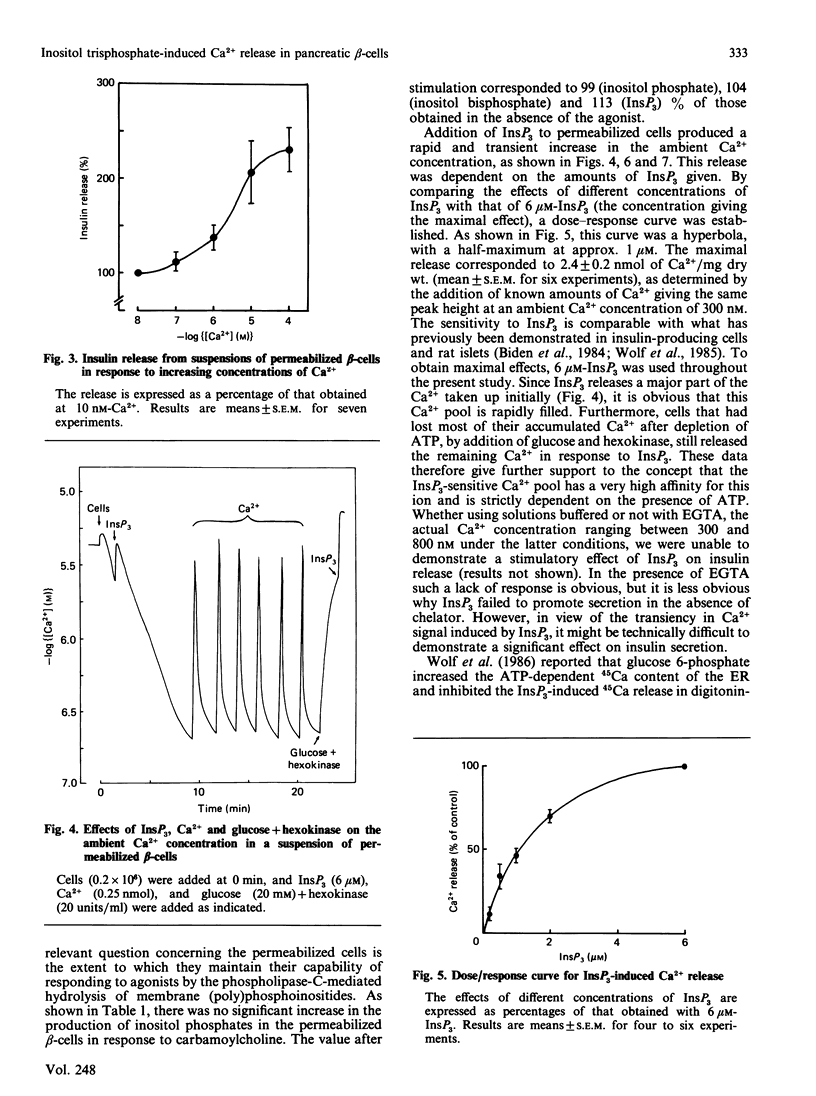
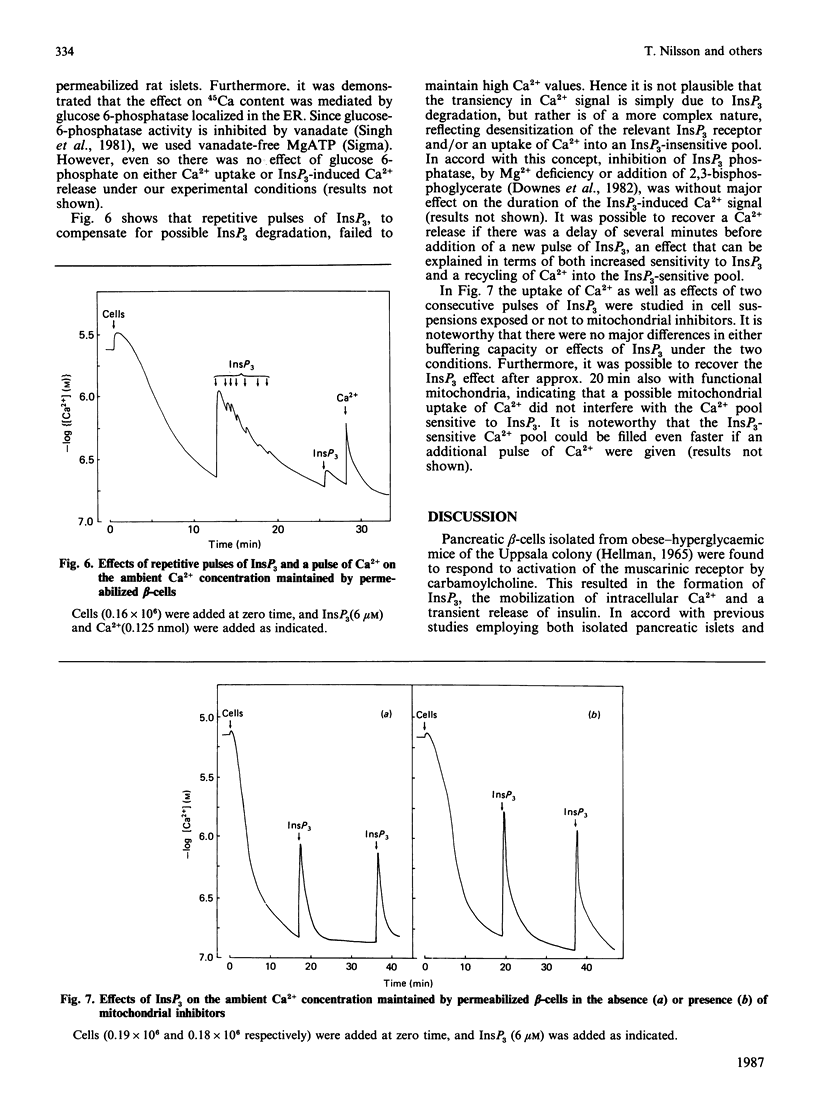
Pancreatic beta-cells isolated from obese-hyperglycaemic mice released intracellular Ca2+ in response to carbamoylcholine, an effect dependent on the presence of glucose. The effective Ca2+ concentration reached was sufficient to evoke a transient release of insulin. When the cells were deficient in Ca2+, the Ca2+ pool sensitive to carbamoylcholine stimulation was equivalent to that released by ionomycin. Unlike intact cells, cells permeabilized by high-voltage discharges failed to generate either inositol 1,4,5-triphosphate (InsP3) or to release Ca2+ after exposure to carbamoylcholine. However, the permeabilized cells released insulin sigmoidally in response to increasing concentrations of Ca2+. Also in the absence of functional mitochondria these cells exhibited a large ATP-dependent buffering of Ca2+, enabling the maintenance of an ambient Ca2+ concentration corresponding to about 150 nM even after several additional pulses of Ca2+. InsP3, maximally effective at 6 microM, promoted a rapid and pronounced release of Ca2+. The InsP3-sensitive Ca2+ pool was rapidly filled and lost its Ca2+ late after ATP depletion. The transient nature of the Ca2+ signal was not overcome by repetitive additions of InsP3. It was possible to restore the response to InsP3 after a delay of approx. 20 min, an effect which had less latency after the addition of Ca2+. These latter findings argue against degradation and/or desensitization as factors responsible for the transiency in InsP3 response. It is suggested that Ca2+ released by InsP3 is taken up by a part of the endoplasmic reticulum (ER) not sensitive to InsP3. On metabolism of InsP3, Ca2+ recycles to the InsP3-sensitive pool, implying that this pool indeed has a very high affinity for the ion. The presence of functional mitochondria did not interfere with the recycling process. The ER in pancreatic beta-cells is of major importance in buffering Ca2+, but InsP3 only modulates Ca2+ transport for a restricted period of time following immediately upon its formation. Thereafter the non-sensitive part of the ER takes over the continuous regulation of Ca2+ cycling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Colca J. R., Wolf B. A., Comens P. G., McDaniel M. L. Protein phosphorylation in permeabilized pancreatic islet cells. Biochem J. 1985 Jun 15;228(3):529–536. doi: 10.1042/bj2280529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. Glucose-stimulated sequestration of Ca2+ in clonal insulin-releasing cells. Evidence for an opposing effect of muscarinic-receptor activation. Biochem J. 1986 Feb 1;233(3):865–870. doi: 10.1042/bj2330865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Gylfe E. Mobilization of different intracellular calcium pools after activation of muscarinic receptors in pancreatic beta-cells. Pharmacology. 1986;32(5):257–267. doi: 10.1159/000138178. [DOI] [PubMed] [Google Scholar]
- Hellman B., Gylfe E., Wesslén N. Inositol 1,4,5-trisphosphate mobilizes glucose-incorporated calcium from pancreatic islets. Biochem Int. 1986 Aug;13(2):383–389. [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Hellman B. The significance of calcium for glucose stimulation of insulin release. Endocrinology. 1975 Aug;97(2):392–398. doi: 10.1210/endo-97-2-392. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Stutchfield J., Howell S. L. Effects of Ca2+ and a phorbol ester on insulin secretion from islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1985 Oct 21;191(1):102–106. doi: 10.1016/0014-5793(85)81002-4. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Kanatsuna T., Lernmark A., Rubenstein A. H., Steiner D. F. Block in insulin release from column-perifused pancreatic beta-cells induced by islet cell surface antibodies and complement. Diabetes. 1981 Mar;30(3):231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Corkey B. E., Matschinsky F. M. Inositol 1,4,5-trisphosphate and the endoplasmic reticulum Ca2+ cycle of a rat insulinoma cell line. J Biol Chem. 1985 Aug 5;260(16):9185–9190. [PubMed] [Google Scholar]
- Prentki M., Janjic D., Biden T. J., Blondel B., Wollheim C. B. Regulation of Ca2+ transport by isolated organelles of a rat insulinoma. Studies with endoplasmic reticulum and secretory granules. J Biol Chem. 1984 Aug 25;259(16):10118–10123. [PubMed] [Google Scholar]
- Singh J., Nordlie R. C., Jorgenson R. A. Vanadate: a potent inhibitor of multifunctional glucose-6-phosphatase. Biochim Biophys Acta. 1981 Dec 18;678(3):477–482. doi: 10.1016/0304-4165(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tamagawa T., Niki H., Niki A. Insulin release independent of a rise in cytosolic free Ca2+ by forskolin and phorbol ester. FEBS Lett. 1985 Apr 22;183(2):430–432. doi: 10.1016/0014-5793(85)80825-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]
- Wolf B. A., Colca J. R., Comens P. G., Turk J., McDaniel M. L. Glucose 6-phosphate regulates Ca2+ steady state in endoplasmic reticulum of islets. A possible link in glucose-induced insulin secretion. J Biol Chem. 1986 Dec 15;261(35):16284–16287. [PubMed] [Google Scholar]
- Wolf B. A., Comens P. G., Ackermann K. E., Sherman W. R., McDaniel M. L. The digitonin-permeabilized pancreatic islet model. Effect of myo-inositol 1,4,5-trisphosphate on Ca2+ mobilization. Biochem J. 1985 May 1;227(3):965–969. doi: 10.1042/bj2270965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Yaseen M. A., Pedley K. C., Howell S. L. Regulation of insulin secretion from islets of Langerhans rendered permeable by electric discharge. Biochem J. 1982 Jul 15;206(1):81–87. doi: 10.1042/bj2060081. [DOI] [PMC free article] [PubMed] [Google Scholar]