Abstract
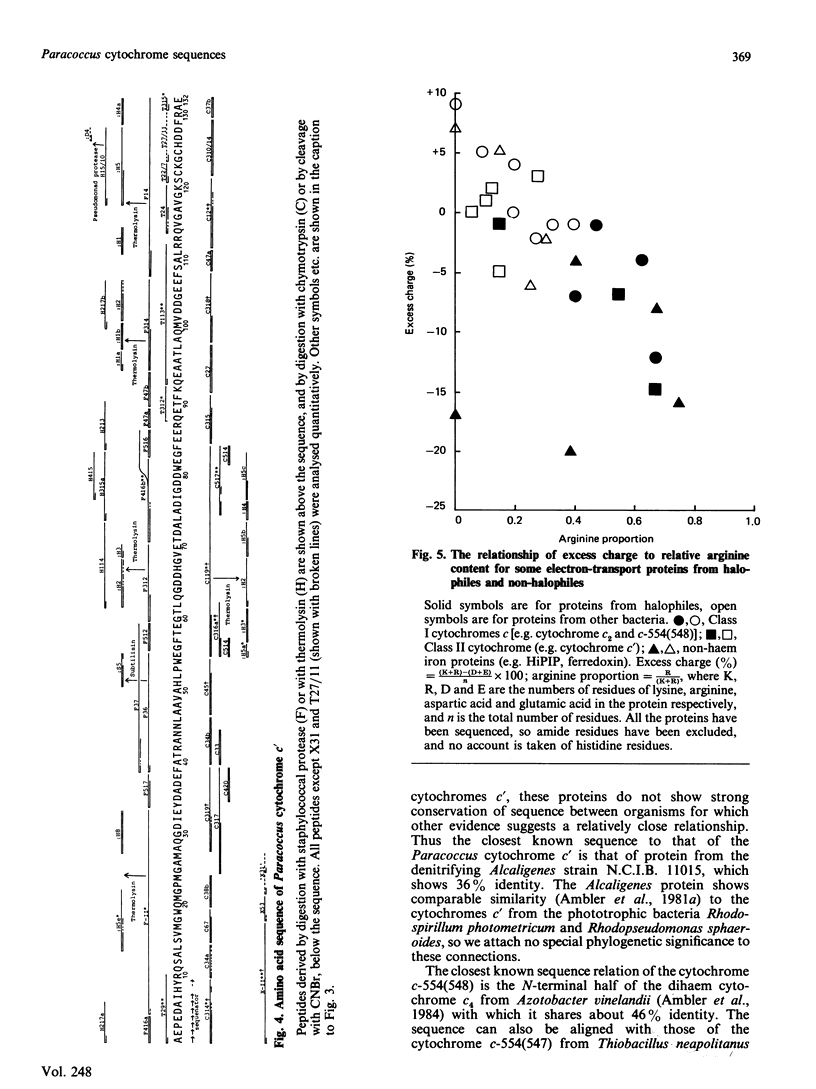
The amino acid sequences of the cytochromes c-554(548) and c' from the moderately halophilic bacterium Paracoccus sp., I.A.M. 203 (= A.T.C.C. 12084, N.C.I.B. 8669) have been determined. Cytochrome c-554(548) consists of a single polypeptide chain of 83 residues, and dimerizes strongly. The most similar protein of known sequence is the N-terminal half of the dihaem cytochrome c4, and other related proteins include the cytochrome c-554(547) of Thiobacillus neapolitanus and the cytochrome c-553 of Desulfovibrio vulgaris. Cytochrome c', which has a single polypeptide chain of 132 residues, is similar in sequence to cytochromes c' from phototrophic and denitrifying bacteria, but only shows about 36% sequence identity to the most similar protein of known sequence. Both of the Paracoccus proteins have a considerable excess of acidic amino acid side chains over basic ones, and a higher proportion of their basic amino acids is arginine than is usual in cytochromes c. Both these characteristics seem to be adaptations to increase the stability of the proteins in an environment of high ionic strength. Detailed evidence for the amino acid sequences of the proteins has been deposited as Supplementary Publication 50140 (24 pp.) at the British Library (Lending Division), Boston Spa, Yorkshire LS23 7BQ, U.K. from which copies are available on prepayment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Dalton H., Meyer T. E., Bartsch R. G., Kamen M. D. The amino acid sequence of cytochrome c-555 from the methane-oxidizing bacterium Methylococcus capsulatus. Biochem J. 1986 Jan 15;233(2):333–337. doi: 10.1042/bj2330333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Melis K., Stout C. D. The amino acid sequence of the dihaem cytochrome c4 from the bacterium Azotobacter vinelandii. Biochem J. 1984 Aug 15;222(1):217–227. doi: 10.1042/bj2220217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Meyer T. E., Bartsch R. G., Kamen M. D. The amino acid sequence of cytochrome c' from the purple sulphur bacterium Chromatium vinosum. Biochem J. 1979 Mar 1;177(3):819–823. doi: 10.1042/bj1770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Kamen M. D., Schichman S. A., Sawyer L. A reassessment of the structure of Paracoccus cytochrome c-550. J Mol Biol. 1981 Apr 5;147(2):351–356. doi: 10.1016/0022-2836(81)90445-9. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Trudinger P. A., Kamen M. D. The amino acid sequence of the cytochrome c-554(547) from the chemolithotrophic bacterium Thiobacillus neapolitanus. Biochem J. 1985 May 1;227(3):1009–1013. doi: 10.1042/bj2271009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Dodson E. J., Norris G. E., Rumball S. V., Waters J. M., Baker E. N. Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanaitis B. C., Aisen P. EPR signal, purple color, and iron binding in porcine uteroferrin. J Biol Chem. 1982 Feb 25;257(4):1855–1859. [PubMed] [Google Scholar]
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Weber P. C., Hardman K. D., Salemme F. R. Structure of ferricytochrome c' from Rhodospirillum molischianum at 1.67 A resolution. J Mol Biol. 1985 Dec 5;186(3):627–643. doi: 10.1016/0022-2836(85)90135-4. [DOI] [PubMed] [Google Scholar]
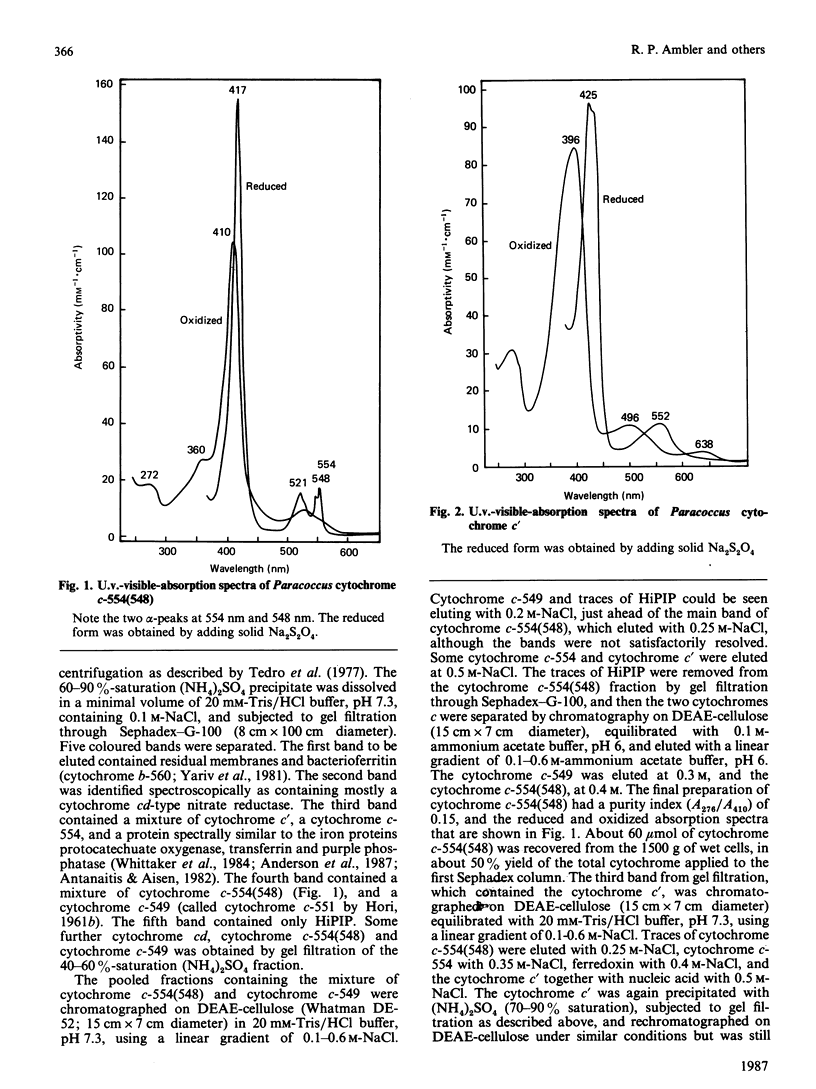
- HORI K. Electron transporting components participating in nitrate and oxygen respirations from a halotolerant Micrococcus. I. Purification and properties of cytochromes b4 (I) and b4 (II). J Biochem. 1961 Nov;50:440–449. doi: 10.1093/oxfordjournals.jbchem.a127473. [DOI] [PubMed] [Google Scholar]
- HORI K. Electron transporting components participating in nitrate and oxygen respirations from a halotolerant Micrococcus. II. Properties of cytochrome c551 and brown protein. J Biochem. 1961 Dec;50:481–485. doi: 10.1093/oxfordjournals.jbchem.a127479. [DOI] [PubMed] [Google Scholar]
- KONO M., TANIGUCHI S. Hydroxylamine reductase of a halotolerant Micrococcus. Biochim Biophys Acta. 1960 Oct 7;43:419–430. doi: 10.1016/0006-3002(60)90466-2. [DOI] [PubMed] [Google Scholar]
- Nakano K., Kikumoto Y., Yagi T. Amino acid sequence of cytochrome c-553 from Desulfovibrio vulgaris Miyazaki. J Biol Chem. 1983 Oct 25;258(20):12409–12412. [PubMed] [Google Scholar]
- Offord R. E. The use of logarithmic plots of electrophoretic mobilities of peptides. Methods Enzymol. 1977;47:51–69. doi: 10.1016/0076-6879(77)47008-3. [DOI] [PubMed] [Google Scholar]
- Rao J. K., Argos P. Structural stability of halophilic proteins. Biochemistry. 1981 Nov 10;20(23):6536–6543. doi: 10.1021/bi00526a004. [DOI] [PubMed] [Google Scholar]
- Tedro S. V., Meyer T. E., Kamen M. D. Primary structure of a high potential iron sulfur protein from a moderately halophilic denitrifying coccus. J Biol Chem. 1977 Nov 10;252(21):7826–7833. [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E., Margoliash E. Amino acid sequence of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Apr 25;251(8):2197–2206. [PubMed] [Google Scholar]
- Verweij H., Christianse K., Van Steveninck J. Ozone-induced formation of O,O'-dityrosine cross-linked in proteins. Biochim Biophys Acta. 1982 Feb 18;701(2):180–184. doi: 10.1016/0167-4838(82)90111-x. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Bartsch R. G., Cusanovich M. A., Hamlin R. C., Howard A., Jordan S. R., Kamen M. D., Meyer T. E., Weatherford D. W., Nguyen huu Xuong Structure of cytochrome c': a dimeric, high-spin haem protein. Nature. 1980 Jul 17;286(5770):302–304. doi: 10.1038/286302a0. [DOI] [PubMed] [Google Scholar]
- Whittaker J. W., Lipscomb J. D., Kent T. A., Münck E. Brevibacterium fuscum protocatechuate 3,4-dioxygenase. Purification, crystallization, and characterization. J Biol Chem. 1984 Apr 10;259(7):4466–4475. [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]


