Abstract
Aims
The sensitivity and specificity of electrodiagnostic parameters in diagnosing carpal tunnel syndrome (CTS) have been reported differently, and this study aims to address this gap.
Methods
This case-control study was conducted on 57 cases with CTS and 58 controls without complaints, such as pain or paresthesia on the median nerve. The main assessed electrodiagnostic parameters were terminal latency index (TLI), residual latency (RL), median ulnar F-wave latency difference (FdifMU), and median sensory latency-ulnar motor latency difference (MSUMLD).
Results
The mean age in cases and controls were 50.7 years (SD 9.9) and 47.9 years (SD 12.1), respectively. The CTS severity was mild in 20 patients (34.4%), moderate in 19 patients (32.8%), and severe in 19 patients (32.8%). The sensitivity and specificity of the electrodiagnostic parameters in diagnosing CTS were as follows: TLI 75.4% and 87.8%; RL 85.9% and 82.5%; FdifMU 87.9% and 82.9%; and MSUMLD 94.8% and 60.0%, respectively.
Conclusion
Our findings indicated that electrodiagnostic parameters are significantly associated with the clinical manifestation of CTS, and are associated with high diagnostic accuracy in CTS diagnosis. However, further studies are required to highlight the role of electrodiagnostic parameters and their combination in CTS detection.
Cite this article: Bone Jt Open 2024;5(10):898–903.
Keywords: Carpal tunnel syndrome, Electromyography, Sensitivity, Specificity, Case-control study, median nerve, paresthesia, clinical manifestation, t-test, nerves, wrist, Tinel sign, electromyography, ulnar nerves
Introduction
Carpal tunnel syndrome (CTS), known as the compression of the median nerve in the carpal tunnel, is the most prevalent focal mononeuropathy.1 The prevalence of CTS in the general population is 8.0%,2 and is frequently present in adults aged between 40 years and 60 years.3 Pain, numbness, tingling, muscle weakness, and decreased hand dexterity function are the most common symptoms of CTS.4 Jobs with forceful hand movements or relying on the hands’ repetitive movements can increase the risk of CTS. The other risk factors included diabetes mellitus, hypothyroidism, trauma, rheumatoid arthritis, tumours, and pregnancy.5
Neuromuscular ultrasound, a quick test that is comfortable for patients, may show imaging evidence suggestive of CTS.6 However, the sensitivity and specificity of this technique have varied in the literature, with sensitivities ranging from 65% to 97%, and the specificities ranging from 73% to 98%.7-9 In addition, presurgical physiological information about the severity of nerve dysfunction at the median nerve or assessment for alternative causes of clinical symptoms is not provided by neuromuscular ultrasound.10
Electrodiagnostic studies, usually used to establish the diagnosis of CTS before a decision for surgery, are supported by reported to establish reliable diagnosis.10-12 The sensitivity of the electrodiagnostic parameters for CTS diagnosis was variable in different studies. Terminal latency index (TLI), residual latency (RL), and median-ulnar F-wave latency difference (FdifMU) can be used as electrodiagnostic parameters identifying abnormalities in the distal segment of motor nerves.12-15 Along with this, some limited studies have shown median sensory latency-ulnar motor latency difference (MSUMLD) to be useful in improving diagnostic accuracy in CTS.16,17
Although TLI, RL, FdifMU, and MSUMLD provide additional information about distal nerve segments, the sensitivity and specificity of these parameters in the diagnosis of CTS have been assessed in limited studies and these four parameters were not compared together.12-15 Therefore, the present study aimed to assess the diagnostic accuracy of all these parameters.
Methods
This case-control study was conducted at the Hamadan University of Medical Sciences, Iran. Cases included patients who were diagnosed with CTS and referred for electromyography. Inclusion criteria for cases were as follows: 1) the presence of two or more of the following positive symptoms: numbness and tingling in the area of the median nerve; night-time paresthesia; pain in the wrist area radiating to the shoulder; positive Phalen test; positive Tinel sign; pain in the wrist area radiating to the shoulder.18 2) Diminished nerve conduction values (below 50 m/s) and/or increased motor latency (above 4 m/s) in a nerve conduction study.18 The control group consisted of patients with no complaints, such as pain or paresthesia on the median nerve tracing, and who were referred for electromyography due to lower limb problems.
Patients with a history of wrist surgeries, carpal tunnel injuries/fractures, radiculopathy and radiculopathy, polyneuropathy, lactation, pregnancy, or rheumatoid arthritis were excluded from the study. The study protocol was approved by the Ethical Review Committee of Hamadan University of Medical Sciences and informed consent was obtained from all participants.
The main variables TLI, RL, FdifMU, and MSUMLD were measured based on electrodiagnostic findings, along with the Tinel sign, Phalen sign, reverse Phalen sign, Thenar atrophy, hand diagram of paresthesia, and Boston score (severity scale and functional scale).19
Based on the hand diagram, CTS was classified into four categories: classic (tingling, numbness, or decreased sensation with or without pain in at least two of digits one, two, or three; palm and dorsum of the hand excluded wrist pain or radiation proximal to the wrist allowed); probable (same as for classic, except palmar symptoms allowed unless confined solely to ulnar aspect); possible (tingling, numbness, decreased sensation, and/or pain in at least one of digits one, two, or thre); or unlikely (no symptoms in digits one, two, or three).19
All participants underwent electrodiagnostic tests using a standard electromyography machine (Nihon Kohden, Japan). Room temperature was maintained at 25°C. To assess the compound’s muscle action potential (CMAP), the active electrode was placed on the abductor pollicis brevis (APB) muscle, the reference electrode was placed on the MCP joint of the thumb, and the median nerve was stimulated at a distance of 8 cm from the active electrode on the wrist.
Sensory nerve action potentials (SNAPs) were obtained antidromically using electrodes placed over the second digit at a distance of 7 cm on the wrist. For the comparison test, electrodes were placed over the fourth digit, and the median nerve and ulnar nerve of the wrist were stimulated individually. Also, for minimum F-wave latencies between the median and ulnar nerves, the stimulator was placed on the proximal side of the wrist.
TLI and RL were calculated using the following formulae:
TLI: (distal nerve conduction distance / distal motor latency) ÷ proximal motor conduction velocity (MCV). The ulnar motor nerve TLI > 0.4 or median motor nerve TLI > 0.31 is considered normal.
RL: distal motor latency − (distal nerve conduction distance / proximal MCV). A value of ulnar RL < 2.1 or median RL < 3.1 is considered normal. Also, the difference between the minimal F-wave latency of the median nerve and the minimal F-wave latency of the ulnar nerve was measured as FdifMU.
Based on the electromyographic findings, patients were categorized into mild, moderate, and severe CTS. Patients with abnormal comparison studies/median sensory nerve abnormalities were considered mild CTS. Prolonged distal motor latency to the APB with normal APB CMAP amplitude was considered moderate CTS and above, plus either reduced median to APB CMAP amplitude and/or abnormal needle electromyography in the Thenar muscles was considered severe CTS.
Statistical analysis
All statistical analyses were performed using Stata software v. 14 (StataCorp, USA). Descriptive data are expressed as mean (SD) or frequency (%) for continuous and categorical data, respectively. Independent-samples t-test was used for comparison of mean values and chi-squared test was used for the comparison of categorical data between case and control groups. Furthermore, the receiver operating characteristic (ROC) curve was used to estimate optimal cutoff points for TLI, RL, FdifMU, and MSUMLD parameters. The optimal cutoff point was estimated with regard to the highest possible logical specificity and sensitivity. Sensitivity, specificity, positive predictive, and negative predictive values with CIs are reported for optimal cutoff points of all parameters. The level of statistical significance was set at a p-value < 0.05.
Results
In this study, 67 patients with CTS and 71 patients without CTS were assessed to select 60 cases and 60 controls. Three cases and two controls were excluded during data gathering due to a lack of informed consent; finally, 57 cases and 58 controls were analyzed (Figure 1). The mean age of cases and controls were 50.7 years (SD 9.9) and 47.9 years (SD 12.1), respectively. Ten cases (17.2%) and 16 controls (28.1%) were male, and 48 cases (82.8%) and 41 controls (71.9%) were female.
Fig. 1.
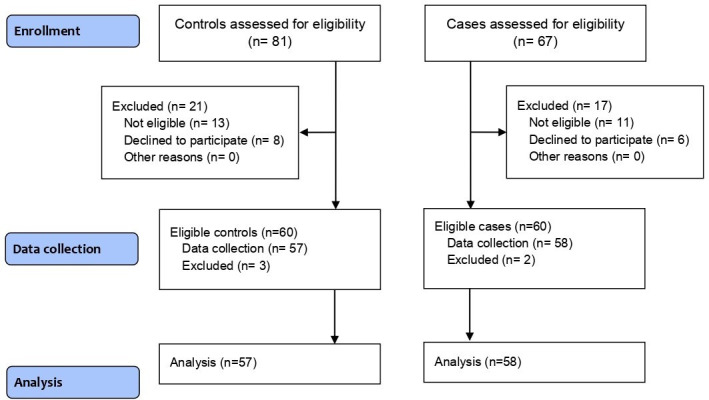
Study flow diagram.
Tinel sign was observed in 48.3% of studied patients. Phalen sign (47 patients, 81.0%) and reverse Phalen sign (48 patients, 82.8%) were most prevalent in the participants. Based on hand diagram findings, CTS in 45 hands (77.6%) was the classic pattern, and in 11 hands (19.0%) was the probable pattern. Also, based on electromyography findings, the severity of CTS in 20 patients (34.4%) was mild 19 patients (32.8%) was moderate, and 19 patients (32.8%) were severe (Table I).
Table I.
Manifestations of studied carpal tunnel syndrome patients.
| Variable | Severity of CTS | Total (n = 58) | p-value* | ||
|---|---|---|---|---|---|
| Mild (n = 20) | Moderate (n = 19) | Severe (n = 19) | |||
| Sign, n (%) | |||||
| Tinel sign | 8 (40.0) | 11 (61.1) | 9 (47.4) | 28 (48.3) | 0.422 |
| Phalen sign | 17 (85.0) | 14 (73.7) | 16 (84.2) | 47 (81.0) | 0.607 |
| Reverse Phalen sign | 16 (80.0) | 15 (78.9) | 17 (89.5) | 48 (82.8) | 0.637 |
| Trophy of Thenar eminence | 0 (0.0) | 1 (5.3) | 4 (21.1) | 5 (8.6) | 0.041 |
| Hand diagram of paresthesia, n (%) | 0.550 | ||||
| Classic | 14 (70.0) | 15 (78.9) | 16 (84.2) | 45 (77.6) | |
| Probable | 6 (30.0) | 3 (15.8) | 2 (10.5) | 11 (19.0) | |
| Unlikely | 0 (0.0) | 1 (5.3) | 1 (5.3) | 2 (3.4) | |
| Mean Boston score (SD) | |||||
| Symptom severity scale | 2.66 (0.55) | 2.58 (0.56) | 2.81 (0.66) | 2.68 (0.60) | 0.510 |
| Functional status scale | 2.72 (0.44) | 2.38 (0.60) | 2.78 (0.51) | 2.6 (0.54) | 0.104 |
Chi-squared test.
CTS, carpal tunnel syndrome.
TLI was significantly higher in cases than controls (0.43 vs 0.32, respectively; p = 0.001, independent-samples t-test). RL, FdifMU, and MSUMLD in cases were significantly lower than controls (p = 0.001, independent-samples t-test). TLI was significantly lower in three CTS categories compared to the control group (p = 0.003, independent-samples t-test). RL was significantly higher in three CTS categories compared to the control group (p = 0.001, independent-samples t-test). MSUMLD in three CTS categories was significantly more than in the control group (p = 0.001, independent-samples t-test). FdifMU in CTS categories was significantly more than in controls (p = 0.002, independent-samples t-test) (Table II).
Table II.
Comparison of electrodiagnostic parameters between case and control groups based on carpal tunnel syndrome severity.
| Variable | Controls (n = 57) | Severity of CTS | Total cases (n = 58) | p-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mild (n = 20) | p-value* | Moderate (n = 19) | p-value* | Severe (n = 19) | p-value* | ||||
| Mean terminal latency index (SD) | 0.43 (0.07) | 0.38 (0.09) | 0.003 | 0.30 (0.05) | 0.001 | 0.28 (0.04) | 0.001 | 0.32 (0.07) | 0.001 |
| Mean residual latency (SD) | 1.85 (0.42) | 2.32 (0.29) | 0.001 | 3.40 (0.86) | 0.001 | 3.86 (0.89) | 0.001 | 3.17 (0.97) | 0.001 |
| Mean MSUMLD (SD) | 0.12 (0.37) | 0.75 (0.37) | 0.001 | 1.43 (0.51) | 0.001 | 1.37 (1.13) | 0.001 | 1.17 (0.79) | 0.001 |
| Mean FdifMU (SD) | -0.27 (1.12) | 0.82 (0.73) | 0.002 | 2.02 (1.34) | 0.001 | 2.50 (2.03) | 0.001 | 1.76 (1.60) | 0.001 |
Independent-sample t-test.
CTS, carpal tunnel syndrome; FdifMU, median ulnar F-wave latency difference; MSUMLD, median sensory latency-ulnar motor latency difference.
Table III shows the ROC and accuracy findings of four electrodiagnostic parameters in detecting CTS. The best cutoff for TLI was below 0.368 with 75.4% sensitivity and 87.8% specificity, with the area under the curve (AUC) of 0.888. The positive predictive value (PPV) and negative predictive value (NPV) of this cutoff were 86.0% and 78.1%, respectively. Below 2.245 with a sensitivity of 85.9% and specificity of 82.5% with AUC of 0.935 was the best cutoff for RL in detecting CTS. The PPV and NPV of this cutoff were 83.3% and 85.4%, respectively. For MSUMLD selected cutoff was below 0.1 with a sensitivity of 94.8% and specificity of 60.0% with an AUC of 0.883. The PPV of this cutoff was 70.5% and NPV was 991.9%. Additionally, FdifMU below 0.5 with a sensitivity of 87.9% and specificity of 82.9% with an AUC of 0.933 was the selected cutoff with PPV 92.7% and NPV 88.3%.
Table III.
Receiver operating characteristic and accuracy findings of four electrodiagnostic parameters in detecting carpal tunnel syndrome.
| Variables | Diagnostic test | ROC curve | p-value* | ||||
|---|---|---|---|---|---|---|---|
| Cut-off | Sensitivity | Specificity | PPV | NPV | AUC | ||
| Terminal Latency Index | ≤ 0.368 | 75.4 (62.2 to 85.9) | 87.7 (76.3 to 94.9) | 86.0 (75.1 to 92.6) | 78.1 (69.2 to 85.0) | 0.88 (0.81 to 0.94) | 0.001 |
| Residual latency | > 2.245 | 85.9 (74.2 to 93.7) | 82.5 (70.1 to 91.3) | 83.3 (73.8 to 89.8) | 85.4 (75.3 to 91.8) | 0.93 (0.87 to 0.97) | 0.001 |
| MSUMLD | > 0.1 | 94.8 (85.6 to 98.9) | 60.0 (45.9 to 73.0) | 70.5 (63.4, 76.7) | 91.9 (78.7 to 84.7) | 0.88 (0.75 to 0.89) | 0.001 |
| FdifMU | 0.5 | 87.9 (76.7 to 95.0) | 92.9 (83.0 to 98.1) | 92.7 (83.1 to 97.0) | 88.3 (79.0 to 93.8) | 0.93 (087 to 0.97) | 0.001 |
Receiver operating characteristic (ROC) curve was used to estimate optimal cutoff points and p-values for studied parameters. The optimal cutoff point was estimated with regard to the highest possible logical specificity and sensitivity.
AUC, area under curve; FdifMU, median ulnar F-wave latency difference; MSUMLD, median sensory latency-ulnar motor latency difference; NPV, negative predictive value; PPV, positive predictive value.
Discussion
CTS diagnosis is based on the history, clinical symptoms, and physical examination, and there is no established standard for its diagnosis. Electrodiagnostic findings are still the main cornerstone in its subjective diagnosis with a variable range of sensitivity and specificity.20 It is essential to establish their validity.
In our study, a “classic” was the most prevalent pattern of the hand diagram of paresthesia. This pattern was associated with CTS severity based on electromyographic findings, but was not statistically significant. These findings were similar to other studies. Paiva Filho et al21 showed that the possible and classic were the most prevalent hand diagrams of paresthesia patterns in patients with CTS, and found that these patterns had a predominance in cases with severe CTS, but were not statistically significant. Moreover, Moradi et al22 confirmed the use of a hand diagram of paresthesia for CTS diagnosis. These findings suggest that the hand diagram of paresthesia findings in combination with electrodiagnostic findings can improve the diagnostic accuracy for CTS severity; however, to prove the diagnostic effect and precision of this method, further studies are needed.
Our study showed no correlation between the electrodiagnostic severity and Boston scores for both the symptom severity scale and functional scale. Similarly, some previous studies reported no correlation between the electrodiagnostic findings and symptom severity,23,24 but other studies reported a correlation between symptom severity and electrodiagnostic findings.25,26 The difference between the studies results can be explained by the different studied patients. The Boston questionnaire is self-reported, and the answers can be affected depending on the patients’ conditions. However, the controversy regarding the relationship between electrodiagnostic findings and symptom severity scale and functional scale indicates the importance of conducting more studies in this field.
We have shown that the mean of TLI, RL, and FdifMU in CTS patients was significantly different from the control groups. Similarly, Uzunkulaoğlu et al15 reported that TLI and RL in CTS patients were significantly higher than controls but FdifMU was significantly lower. Alcan et al16 showed that the mean of TLI and RL in patients were significantly higher than controls. Furthermore, other studies have demonstrated that the mean TLI in CTS patients was significantly lower than in controls.13,14,27 These findings highlight the abnormalities of electrodiagnostic parameters in CTS patients that can be useful as diagnostic tests. Several studies have been conducted to evaluate the diagnostic accuracy of electrodiagnostic parameters, and reported different values for the sensitivity and specificity of the variables.13,15,16,27 Additionally, in the study by Uzunkulaoglu et al15 TLI had a sensitivity of 65.6% and specificity of 73.8%; RL had a sensitivity of 72.5% and FdifMU a sensitivity of 63.1%. Alcan et al16 reported a sensitivity of 77.9% and 89.7% for TLI and RL, respectively. In the Vahdatpour et al13 study, the sensitivity of TLI was 82%; similarly, Shakouri et al27 reported that TLI had a sensitivity of 81.5%. In our study, we found that TLI had a sensitivity of 75.4%, RL a sensitivity of 85.9%, and FdifMU a sensitivity of 87.9%. As the findings show, the sensitivity of electrodiagnostic parameters is variable between studies. This may have been due to the different distribution of CTS severity among studies’ participating patients. These findings show that these parameters can be useful in the diagnosis of CTS, although conducting a systematic review and meta-analysis could combine the results of the studies and clarify the diagnostic value of these parameters in the diagnosis of CTS and its severity.
MSUMLD was shown to be useful in the diagnosis of the CTS in limited studies.16,17 In the Bodofsky et al17 study, more than 80% of CTS patients had abnormal MSUMLD. The study by Alcan et al16 on 525 CTS patients and 121 controls reported that MSUMLD had a sensitivity of 86.4% and a specificity of 89.3% in the diagnosis of CTS. Similarly, our study on 58 CTS patients and 57 controls showed that MSUMLD had a sensitivity of 94.8% and a specificity of 60%. However, the sensitivity of MSUMLD in our study is higher compared with Alcan et al16. This may be due to our smaller study size or different distribution of CTS severity among the study patients. In our study, 32.7% of patients had severe CTS, but in Alcan et al16 only 6.3% of patients had severe CTS. Diagnostic methods in severe cases are more sensitive than mild cases. Generally, these findings show a very high sensitivity of MSUMLD for CTS, given that it does not require mid-palm stimulation, is done in less time, and leads to increasing patient satisfaction. Additionally, since there is little evidence in this field, it is necessary to conduct more studies.
The present study had some limitations. First, the study participants were recruited from a single clinic affiliated with Hamadan University of Medical Sciences, Iran. This may limit the generalizability of the findings to other populations or healthcare settings. Second, the sample size was also relatively small, which may further impact the generalizability of the results. On the other hand, it may have reduced the statistical power of the our study in recognizing meaningful relationships. Third, the study used electrodiagnostic tests to measure parameters. While these tests are commonly used in diagnosing CTS, they have limitations and potential sources of error. Variability in electrode placement, stimulation techniques, and interpretation of results can introduce measurement errors and affect the reliability of the findings. Finally, the assessors of the electrodiagnostic tests and the clinical assessments were not blinded to the case-control status of the participants. Lack of blinding might introduce potential bias in the assessment and interpretation of the results.
In conclusion, our study demonstrated that electrodiagnostic parameters, including TLI, RL, FdifMU, and MSUMLD are significantly associated with the clinical manifestation of CTS, and these parameters with good diagnostic accuracy values show their utility in improving CTS diagnosis. However, further research are required to highlight the role of electrodiagnostic parameters and their combination for early and severe detection of CTS.
Take home message
- Electrodiagnostic parameters in patients with carpal tunnel syndrome are significantly different from healthy people.
- These parameters can therefore be used to improve carpal tunnel syndrome diagnosis.
Author contributions
S. Mazaheri: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing, Visualization
J. Poorolajal: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing
A. Mazaheri: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
The authors have no conflicts of interest to declare.
Data sharing
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
Acknowledgements
We extend our appreciation to the Vice-Chancellor for Research and Technology of the Hamadan University of Medical Sciences, Iran, for approving this study.
Ethical review statement
This study approval was obtained from the Ethical Committee of Hamadan University of Medical Sciences (No.IR.UMSHA.REC.1400.579). The study was conducted in accordance with the Declaration of Helsinki.
© 2024 Mazaheri et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Shahir Mazaheri, Email: sh.mazaheri@umsha.ac.ir.
Jalal Poorolajal, Email: poorolajal@umsha.ac.ir.
Alireza Mazaheri, Email: Mazaheri.alireza76@gmail.com.
Data Availability
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
References
- 1. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med. 2002;346(23):1807–1812. doi: 10.1056/NEJMcp013018. [DOI] [PubMed] [Google Scholar]
- 2. Luckhaupt SE, Dahlhamer JM, Ward BW, Sweeney MH, Sestito JP, Calvert GM. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 National Health Interview Survey. Am J Ind Med. 2013;56(6):615–624. doi: 10.1002/ajim.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibrahim I, Khan WS, Goddard N, Smitham P. Carpal tunnel syndrome: a review of the recent literature. Open Orthop J. 2012;6:69–76. doi: 10.2174/1874325001206010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 5. Newington L, Harris EC, Walker-Bone K. Carpal tunnel syndrome and work. Best Pract Res Clin Rheumatol. 2015;29(3):440–453. doi: 10.1016/j.berh.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaki HA, Shaban E, Salem W, et al. A comparative analysis between ultrasound and electromyographic and nerve conduction studies in diagnosing carpal tunnel syndrome (CTS): a systematic review and meta-analysis. Cureus. 2022;14(10):e30476. doi: 10.7759/cureus.30476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mhoon JT, Juel VC, Hobson-Webb LD. Median nerve ultrasound as a screening tool in carpal tunnel syndrome: correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle Nerve. 2012;46(6):871–878. doi: 10.1002/mus.23426. [DOI] [PubMed] [Google Scholar]
- 8. Visser LH, Smidt MH, Lee ML. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2008;79(1):63–67. doi: 10.1136/jnnp.2007.115337. [DOI] [PubMed] [Google Scholar]
- 9. Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. 2009;37(3):125–131. doi: 10.1002/jcu.20551. [DOI] [PubMed] [Google Scholar]
- 10.Kothari MJ. UpToDate; 2019. [12 September 2024]. Carpal tunnel syndrome: clinical manifestations and diagnosis.https://medilib.ir/uptodate/show/5288 date last. accessed. [Google Scholar]
- 11. Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44(4):597–607. doi: 10.1002/mus.22208. [DOI] [PubMed] [Google Scholar]
- 12. Leventoglu A, Kuruoglu R. Do electrophysiological findings differ according to the clinical severity of carpal tunnel syndrome? J Neurol Sci. 2006;23:272–278. [Google Scholar]
- 13. Vahdatpour B, Khosrawi S, Chatraei M. The role of median nerve terminal latency index in the diagnosis of carpal tunnel syndrome in comparison with other electrodiagnostic parameters. Adv Biomed Res. 2016;5:110. doi: 10.4103/2277-9175.183671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khosrawi S, Dehghan F. Determination of the median nerve residual latency values in the diagnosis of carpal tunnel syndrome in comparison with other electrodiagnostic parameters. J Res Med Sci. 2013;18(11):934–938. [PMC free article] [PubMed] [Google Scholar]
- 15. Uzunkulaoğlu A, Afsar SI, Tepeli B. Terminal latency index, residual latency, and median-ulnar F-wave latency difference in carpal tunnel syndrome. Ann Indian Acad Neurol. 2019;22(2):175–179. doi: 10.4103/aian.AIAN_276_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alcan V, Zinnuroğlu M, Kaymak Karataş G, Bodofsky E. Comparison of interpolation methods in the diagnosis of carpal tunnel syndrome. Balkan Med J. 2018;35(5):378–383. doi: 10.4274/balkanmedj.2017.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bodofsky EB, Wu KD, Campellone JV, Greenberg WM, Tomaio AC. A sensitive new median-ulnar technique for diagnosing mild carpal tunnel syndrome. Electromyogr Clin Neurophysiol. 2005;45(3):139–144. [PubMed] [Google Scholar]
- 18. Srikanteswara PK, Cheluvaiah JD, Agadi JB, Nagaraj K. The relationship between nerve conduction study and clinical grading of carpal tunnel syndrome. J Clin Diagn Res. 2016;10(7):C13–8. doi: 10.7860/JCDR/2016/20607.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17(11):1495–1498. [PubMed] [Google Scholar]
- 20. Genova A, Dix O, Saefan A, Thakur M, Hassan A. Carpal tunnel syndrome: a review of literature. Cureus. 2020;12(3):e7333. doi: 10.7759/cureus.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paiva Filho HR, Costa AC, Paiva VGN, Santos DA, Chakkour I. Is there an association between the hand diagram and electrodiagnostic testing for carpal tunnel syndrome? Rev Bras Ortop (Sao Paulo) 2021;56(1):74–77. doi: 10.1055/s-0040-1713763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moradi A, Mellema JJ, Oflazoglu K, Isakov A, Ring D, Vranceanu A-M. The relationship between catastrophic thinking and hand diagram areas. J Hand Surg Am. 2015;40(12):2440–2446. doi: 10.1016/j.jhsa.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 23. Chan L, Turner JA, Comstock BA, et al. The relationship between electrodiagnostic findings and patient symptoms and function in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88(1):19–24. doi: 10.1016/j.apmr.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 24. Koo JH, Bae J-Y, Lee K, Park HS. Correlation between electrodiagnostic severity and Boston carpal tunnel questionnaire in surgically treated carpal tunnel syndrome patients. Acta Orthop Traumatol Turc. 2023;57(6):357–360. doi: 10.5152/j.aott.2023.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. You H, Simmons Z, Freivalds A, Kothari MJ, Naidu SH. Relationships between clinical symptom severity scales and nerve conduction measures in carpal tunnel syndrome. Muscle Nerve. 1999;22(4):497–501. doi: 10.1002/(sici)1097-4598(199904)22:4<497::aid-mus11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26. Taewon Jeong DSA. Efficiency of the boston questionnaire in carpal tunnel syndrome: comparing scores with provocation tests and electrophysiological studies. J Korean Soc Surg Hand. 2011;16 [Google Scholar]
- 27. Shakouri SK, Eftekhar Sadat B, Salekzamani Y, Taheragdam A, Abedi F. Determination of terminal latency index in carpal tunnel syndrome. Med J Tabriz Univ Med Sci. 2006;28:3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.


