Abstract
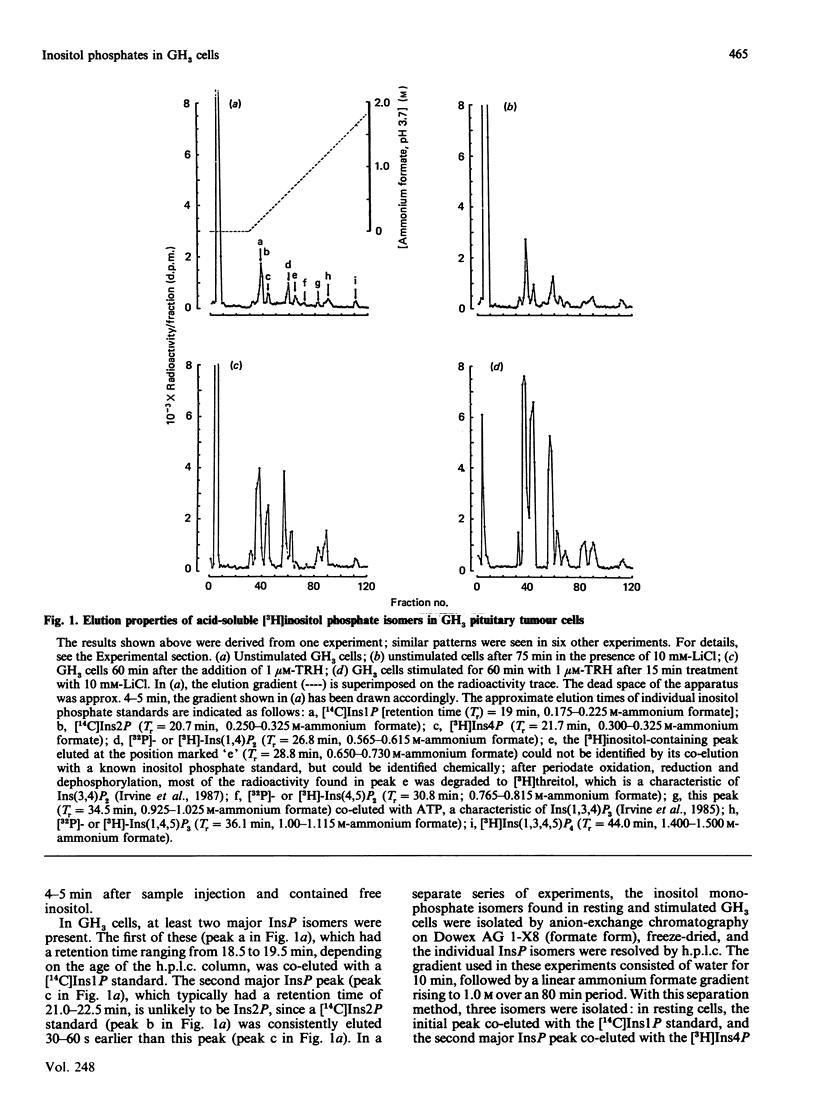
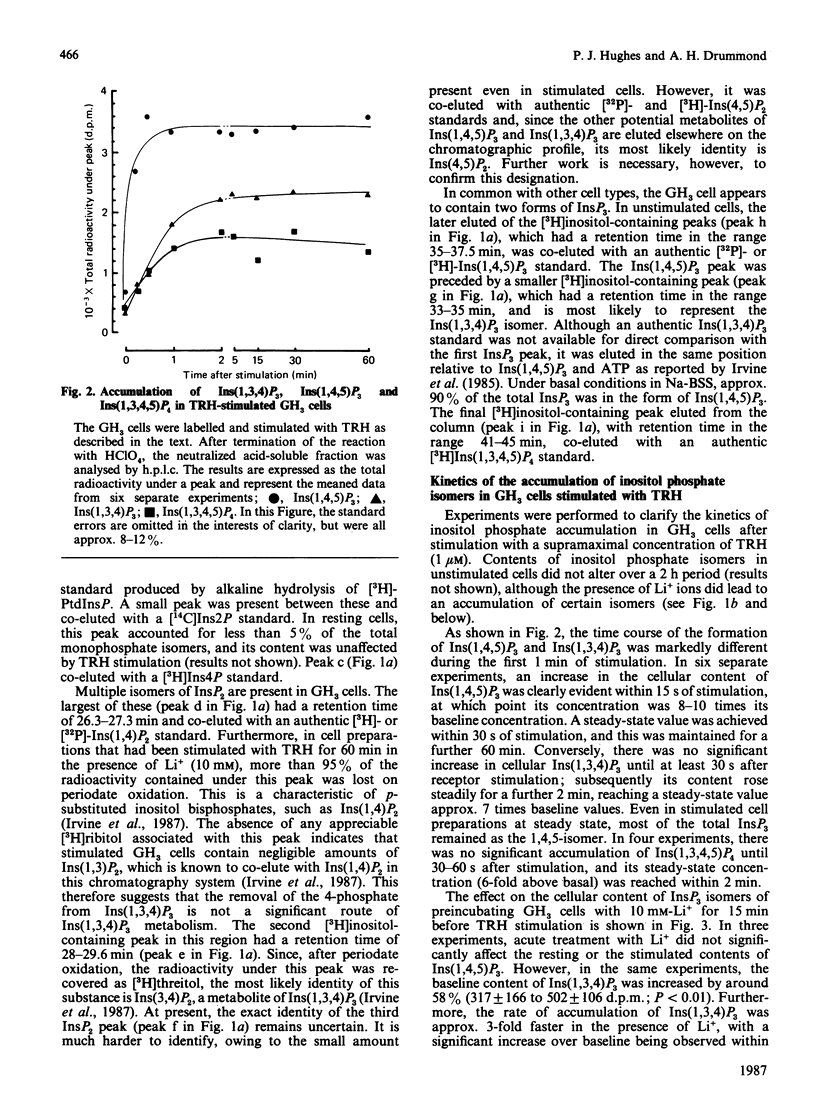
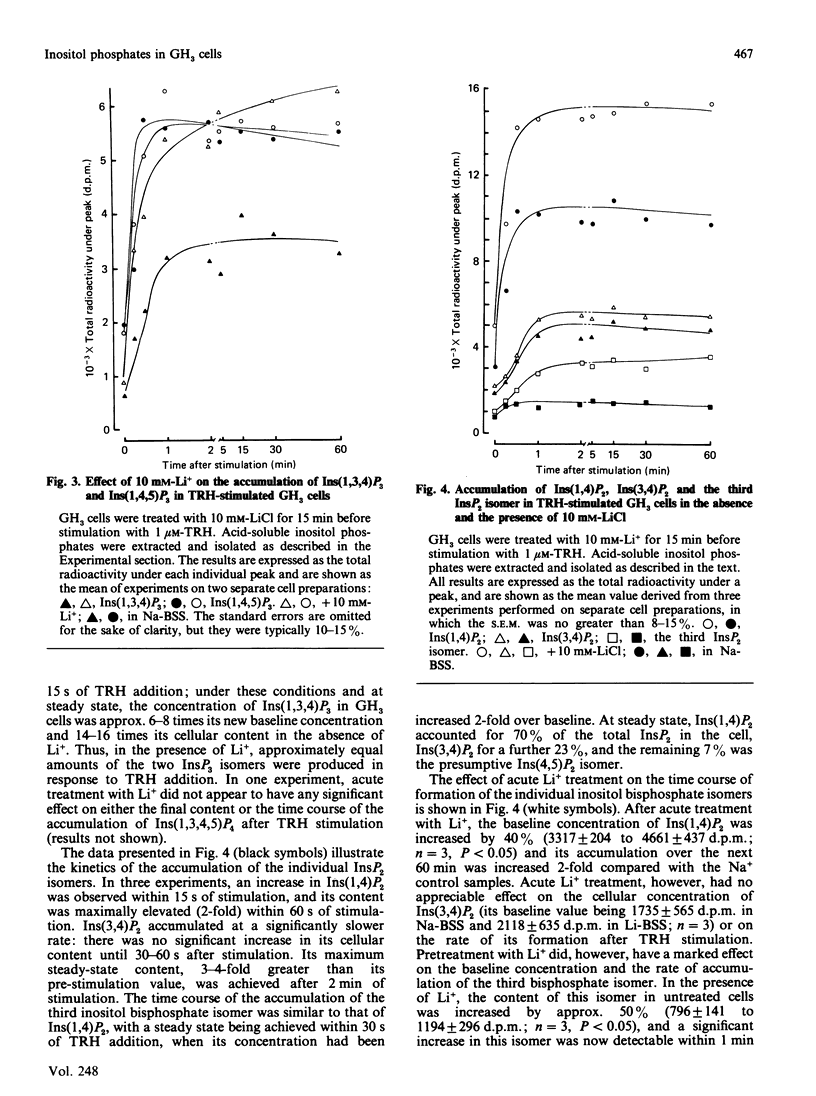
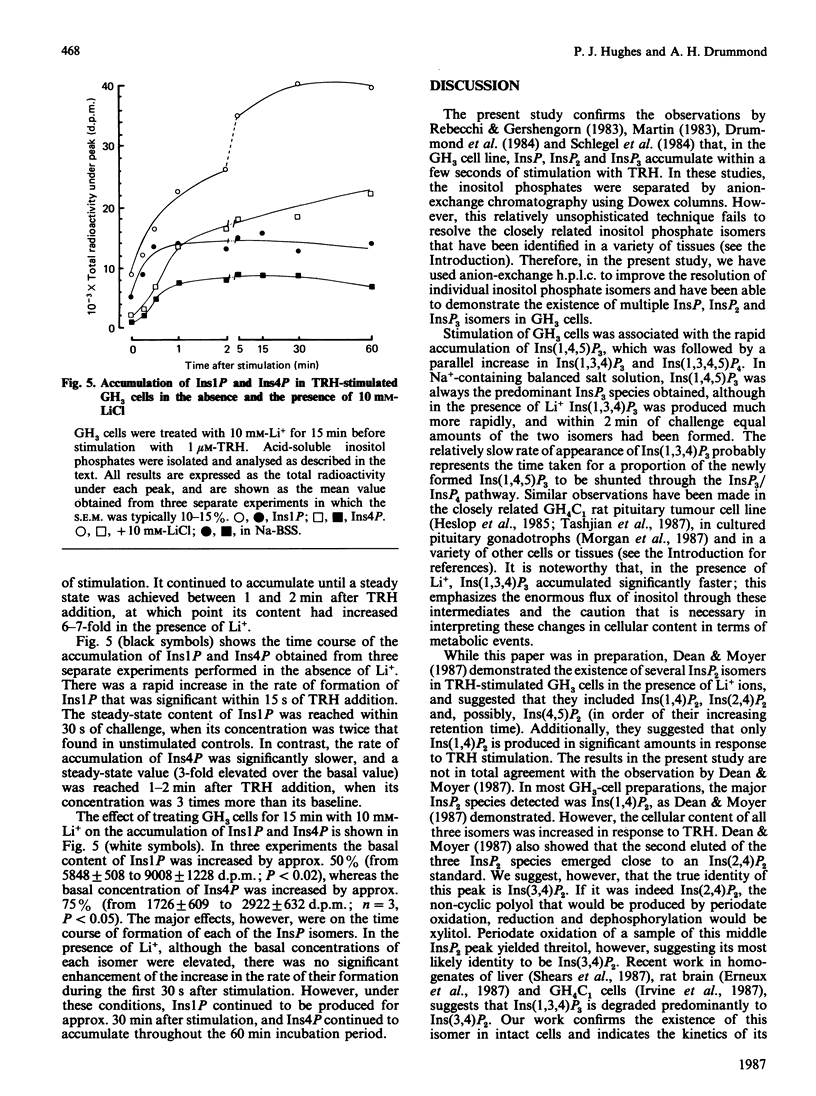
With a h.p.l.c. system, the inositol mono-, bis- and tris-phosphate isomers found in [3H]inositol-labelled GH3 cells were resolved and identified. These cells possess at least ten distinct [3H]inositol-containing substances when acid-soluble extracts are analysed by anion-exchange h.p.l.c. These substances were identified by their co-elution with known inositol phosphate standards and, to a limited extent, by examining their chemical structure. Two major inositol monophosphate (InsP) isomers were identified, namely Ins1P and Ins4P, both of which accumulate after stimulation with the hypothalamic releasing factor (TRH) (thyrotropin-releasing hormone). Three inositol bisphosphate (InsP2) isomers were resolved, of which two were positively identified, i.e. Ins(1,4)P2 and Ins(3,4)P2. TRH treatment increases both of these isomers, with Ins(1,4)P2 being produced at a faster rate than Ins(3,4)P2. The third InsP2 isomer has yet to be fully identified, although it is co-eluted with an Ins(4,5)P2 standard. This third InsP2 is also increased after TRH stimulation. In common with other cell types, the GH3 cell contains two inositol trisphosphate (InsP3) isomers: Ins(1,4,5)P3, which accumulates rapidly, and Ins(1,3,4)P3, which is formed more slowly. The latter substance appears simultaneously with its precursor, inositol 1,3,4,5-tetrakisphosphate. We also examined the effects of acute Li+ treatment on the rates of accumulation of these isomers, and demonstrated that Li+ augments TRH-mediated accumulation of Ins1P, Ins4P, Ins(1,4)P2, the presumed Ins(4,5)P2 and Ins(1,3,4)P3. These results suggest that the effects of Li+ on inositol phosphate metabolism are more complex than was originally envisaged, and support work carried out by less sophisticated chromatographic analysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann K. E., Gish B. G., Honchar M. P., Sherman W. R. Evidence that inositol 1-phosphate in brain of lithium-treated rats results mainly from phosphatidylinositol metabolism. Biochem J. 1987 Mar 1;242(2):517–524. doi: 10.1042/bj2420517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Morgan R. O., Catt K. J. Angiotensin-stimulated production of inositol trisphosphate isomers and rapid metabolism through inositol 4-monophosphate in adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9323–9327. doi: 10.1073/pnas.83.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux A., Erneux C., Moreau C., Dumont J. E. Enzymic dephosphorylation of D-myo-inositol 1,4-bisphosphate in rat brain. Biochem J. 1987 Feb 15;242(1):193–198. doi: 10.1042/bj2420193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Drummond A. H. Inositol lipid metabolism and signal transduction in clonal pituitary cells. J Exp Biol. 1986 Sep;124:337–358. doi: 10.1242/jeb.124.1.337. [DOI] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. The dephosphorylation pathway of D-myo-inositol 1,3,4,5-tetrakisphosphate in rat brain. Biochem J. 1987 Nov 1;247(3):635–639. doi: 10.1042/bj2470635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Lewis D. G., Greer T. L. Thyrotropin-releasing hormone-receptor interaction in GH3 pituitary cells. Endocrinology. 1980 Mar;106(3):1000–1005. doi: 10.1210/endo-106-3-1000. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Phosphatidylinositol 4,5-bisphosphate turnover is transient while phosphatidylinositol turnover is persistent in thyrotropin-releasing hormone-stimulated rat pituitary cells. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8540–8544. doi: 10.1073/pnas.83.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Bansal V. S., Majerus P. W. Pathway for inositol 1,3,4-trisphosphate and 1,4-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2170–2174. doi: 10.1073/pnas.84.8.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H. Thyrotropin-releasing hormone stimulates rapid breakdown of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate in GH3 pituitary tumor cells. Mol Pharmacol. 1984 Mar;25(2):193–200. [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Morgan R. O., Chang J. P., Catt K. J. Novel aspects of gonadotropin-releasing hormone action on inositol polyphosphate metabolism in cultured pituitary gonadotrophs. J Biol Chem. 1987 Jan 25;262(3):1166–1171. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Leung J. T., Hajek S. V., Winokur T., Melman M. Acute stimulated hormone release from cultured GH3 pituitary cells. Endocrinology. 1978 Oct;103(4):1245–1252. doi: 10.1210/endo-103-4-1245. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. R. Polyphosphoinositide hydrolysis by phospholipase C is accelerated by thyrotropin releasing hormone (TRH) in clonal rat pituitary cells (GH3 cells). FEBS Lett. 1984 Mar 12;168(1):54–60. doi: 10.1016/0014-5793(84)80205-7. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W. R., Munsell L. Y., Gish B. G., Honchar M. P. Effects of systemically administered lithium on phosphoinositide metabolism in rat brain, kidney, and testis. J Neurochem. 1985 Mar;44(3):798–807. doi: 10.1111/j.1471-4159.1985.tb12886.x. [DOI] [PubMed] [Google Scholar]
- Siess W. Evidence for the formation of inositol 4-monophosphate in stimulated human platelets. FEBS Lett. 1985 Jun 3;185(1):151–156. doi: 10.1016/0014-5793(85)80760-2. [DOI] [PubMed] [Google Scholar]
- Siess W., Stifel M., Binder H., Weber P. C. Activation of V1-receptors by vasopressin stimulates inositol phospholipid hydrolysis and arachidonate metabolism in human platelets. Biochem J. 1986 Jan 1;233(1):83–91. doi: 10.1042/bj2330083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- TOMLINSON R. V., BALLOU C. E. Complete characterization of the myo-inositol polyphosphates from beef brain phosphoinositide. J Biol Chem. 1961 Jul;236:1902–1906. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Heslop J. P., Berridge M. J. Subsecond and second changes in inositol polyphosphates in GH4C1 cells induced by thyrotropin-releasing hormone. Biochem J. 1987 Apr 1;243(1):305–308. doi: 10.1042/bj2430305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J., Wolf B. A., McDaniel M. L. Glucose-induced accumulation of inositol trisphosphates in isolated pancreatic islets. Predominance of the 1,3,4-isomer. Biochem J. 1986 Jul 1;237(1):259–263. doi: 10.1042/bj2370259. [DOI] [PMC free article] [PubMed] [Google Scholar]


