Abstract
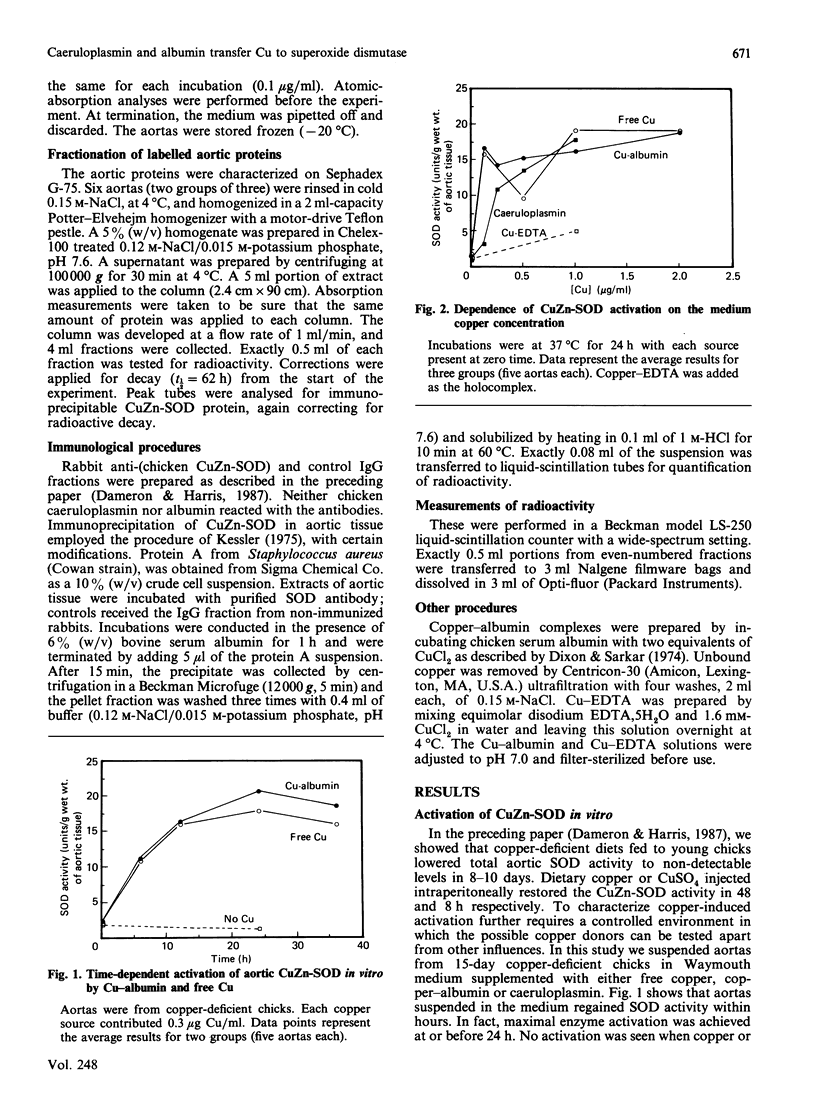
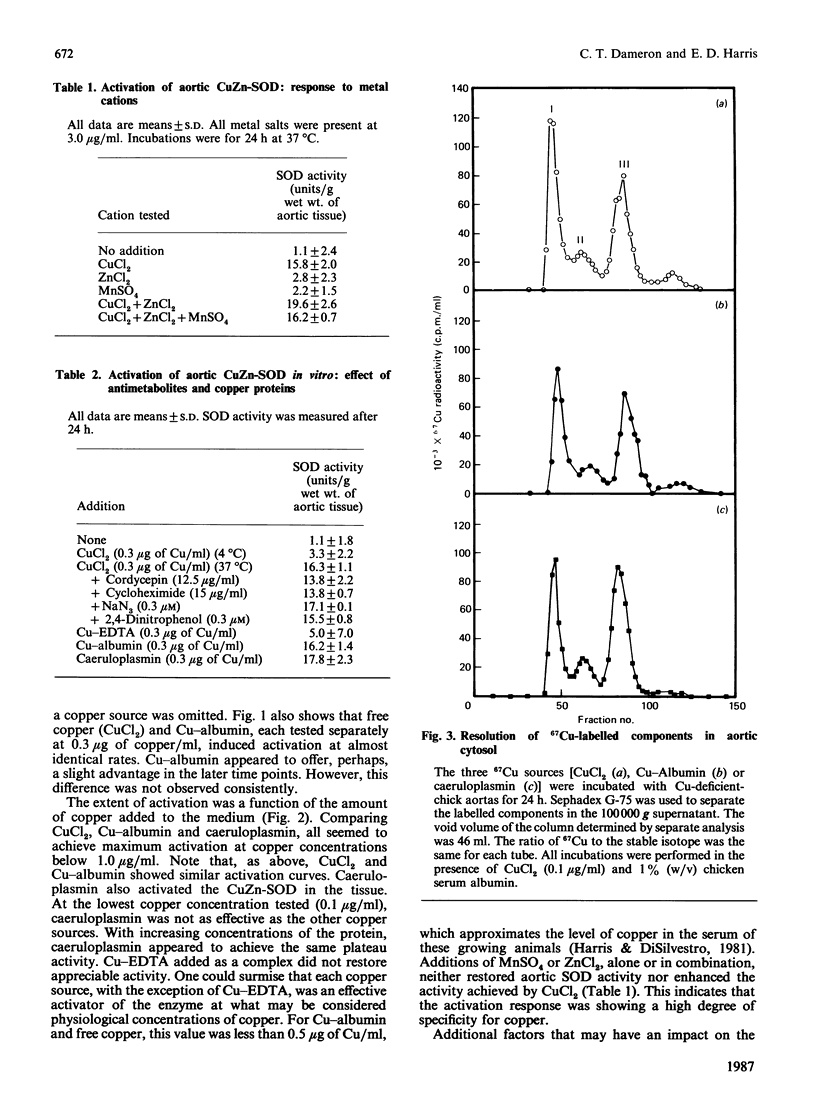
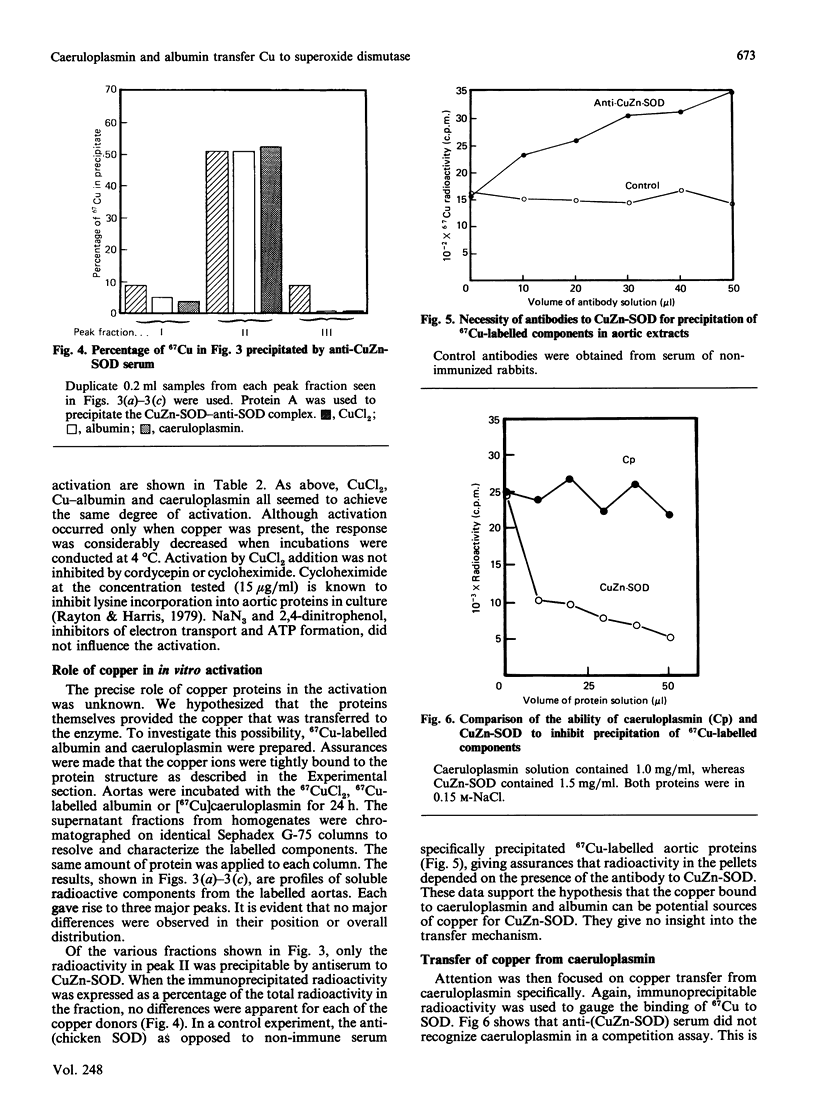
Caeruloplasmin and albumin were compared as potential donors of copper to Cu2Zn2-superoxide dismutase (CuZn-SOD) in culture. Aortas from 15-day copper-deficient chicks were suspended in oxygenated, serum-free, Waymouth medium (752/1) for 24 h. SOD activity was restored when the medium was supplemented with CuCl2, a copper-albumin complex or caeruloplasmin, all present at a level equivalent to 5 microM-copper. Activation did not occur at 4 degrees C or with Cu-EDTA as the supplement. Mn2+ and Zn2+, alone or in combination, did not activate nor enhance the activation achieved by CuCl2. The activation with CuCl2 was not inhibited by cycloheximide or cordycepin. [67Cu]Caeruloplasmin and albumin when added to the medium transferred radioactive copper to at least three cytosolic protein fractions, one of which was determined by immunoprecipitation to be CuZn-SOD. The transfer of 67Cu from caeruloplasmin was inhibited by increasing amounts of unlabelled caeruloplasmin; disodium EDTA (1.0 mM) had no effect on the transfer of copper from caeruloplasmin. These data show that aortic SOD activity, suppressed in copper deficiency, can be restored by incubating the aortas in culture medium supplemented with copper salts. In this system, caeruloplasmin and Cu-albumin appear equally capable of activating aortic CuZn-SOD. Moreover, the transfer of copper into the enzyme structure appears to be the primary event restoring catalytic activity to the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Barnes G., Frieden E. Ceruloplasmin receptors of erythrocytes. Biochem Biophys Res Commun. 1984 Nov 30;125(1):157–162. doi: 10.1016/s0006-291x(84)80348-4. [DOI] [PubMed] [Google Scholar]
- Dameron C. T., Harris E. D. Regulation of aortic CuZn-superoxide dismutase with copper. Effects in vivo. Biochem J. 1987 Dec 15;248(3):663–668. doi: 10.1042/bj2480663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disilvestro R. A., Harris E. D. Purification and partial characterization of ceruloplasmin from chicken serum. Arch Biochem Biophys. 1985 Sep;241(2):438–446. doi: 10.1016/0003-9861(85)90568-5. [DOI] [PubMed] [Google Scholar]
- Dixon J. W., Sarkar B. Isolation, amino acid sequence and copper(II)-binding properties of peptide (1-24) of dog serum albumin. J Biol Chem. 1974 Sep 25;249(18):5872–5877. [PubMed] [Google Scholar]
- Giclas P. C., Manthei U., Strunk R. C. The acute phase response of C3, C5, ceruloplasmin, and C-reactive protein induced by turpentine pleurisy in the rabbit. Am J Pathol. 1985 Jul;120(1):146–156. [PMC free article] [PubMed] [Google Scholar]
- Harris E. D. Copper-induced activation of aortic lysyl oxidase in vivo. Proc Natl Acad Sci U S A. 1976 Feb;73(2):371–374. doi: 10.1073/pnas.73.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., DiSilvestro R. A. Correlation of lysyl oxidase activation with the p-phenylenediamine oxidase activity (ceruloplasmin) in serum. Proc Soc Exp Biol Med. 1981 Apr;166(4):528–531. doi: 10.3181/00379727-166-41102. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Gonnerman W. A., Savage J. E., O'Dell B. L. Connective tissue amine oxidase. II. Purification and partial characterization of lysyl oxidase from chick aorta. Biochim Biophys Acta. 1974 Apr 25;341(2):332–344. doi: 10.1016/0005-2744(74)90226-5. [DOI] [PubMed] [Google Scholar]
- Jenkinson S. G., Lawrence R. A., Grafton W. D., Gregory P. E., McKinney M. A. Enhanced pulmonary toxicity in copper-deficient rats exposed to hyperoxia. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 1):170–177. doi: 10.1016/0272-0590(84)90117-9. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. Ceruloplasmin receptors in liver cell suspensions are limited to the endothelium. Exp Cell Res. 1984 Nov;155(1):232–240. doi: 10.1016/0014-4827(84)90784-5. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. Identification of ceruloplasmin receptors on the surface of human blood monocytes, granulocytes, and lymphocytes. Exp Hematol. 1985 Sep;13(8):806–810. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Marceau N., Aspin N. The intracellular distribution of the radiocopper derived from ceruloplasmin and from albumin. Biochim Biophys Acta. 1973 Dec 6;328(2):338–350. doi: 10.1016/0005-2795(73)90267-5. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mohanakrishnan P., Chignell C. F. Ion-specific electrode study of copper binding to serum albumins. J Pharm Sci. 1982 Oct;71(10):1180–1182. doi: 10.1002/jps.2600711028. [DOI] [PubMed] [Google Scholar]
- Orena S. J., Goode C. A., Linder M. C. Binding and uptake of copper from ceruloplasmin. Biochem Biophys Res Commun. 1986 Sep 14;139(2):822–829. doi: 10.1016/s0006-291x(86)80064-x. [DOI] [PubMed] [Google Scholar]
- Paynter D. I., Moir R. J., Underwood E. J. Changes in activity of the Cu-Zn superoxide dismutase enzyme in tissues of the rat with changes in dietary copper. J Nutr. 1979 Sep;109(9):1570–1576. doi: 10.1093/jn/109.9.1570. [DOI] [PubMed] [Google Scholar]
- Rayton J. K., Harris E. D. Induction of lysyl oxidase with copper. Properties of an in vitro system. J Biol Chem. 1979 Feb 10;254(3):621–626. [PubMed] [Google Scholar]
- Schmitt R. C., Darwish H. M., Cheney J. C., Ettinger M. J. Copper transport kinetics by isolated rat hepatocytes. Am J Physiol. 1983 Feb;244(2):G183–G191. doi: 10.1152/ajpgi.1983.244.2.G183. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutase of Photobacterium leiognathi. Isolation and sequence of the gene and evidence for a precursor form. J Biol Chem. 1987 Feb 5;262(4):1882–1887. [PubMed] [Google Scholar]
- Stevens M. D., DiSilvestro R. A., Harris E. D. Specific receptor for ceruloplasmin in membrane fragments from aortic and heart tissues. Biochemistry. 1984 Jan 17;23(2):261–266. doi: 10.1021/bi00297a014. [DOI] [PubMed] [Google Scholar]
- Terao T., Owen C. A., Jr Copper in supernatant fractions of various rat tissues. Studies with 67Cu. Mayo Clin Proc. 1974 Jun;49(6):376–381. [PubMed] [Google Scholar]
- Terao T., Owen C. A., Jr Effects of copper deficiency and copper loading on 67Cu in supernatants of rat organs. Tohoku J Exp Med. 1976 Nov;120(3):209–217. doi: 10.1620/tjem.120.209. [DOI] [PubMed] [Google Scholar]


